Keywords |
| Skeletal muscle, Appendicular skeletal muscle mass, Young adults, Risk factors, Rural area |
Introduction |
| Skeletal mass loss is a part of the ageing process after
reaching a peak in early adulthood, at about 40-45
years of age, it progressively declines about 8% per
decade until the age of 70 and about 15% afterward [1,
2] but also is a consequence of acquired conditions
including neurodegenerative diseases, endocrinological
abnormalities, inadequate nutrition or malabsorption,
cachexia and disuse such as in physical inactivity [1].
Moreover, the ageing process is associated with decreased
muscle strength and physical performance that are related
to an increase of the prevalence of sarcopenia. Sarcopenia is one of the geriatric syndromes that is an age-related
decline in skeletal mass and muscle function which is
related to physical disability, falls, poor quality of life,
adverse metabolic outcomes, increases in healthcare costs
and mortality in older adults [3]. According to the consensus
of the Asian Working Group for Sarcopenia (AWGS),
the diagnosis of this condition requires measurements of
muscle mass, muscle strength, and physical performance
[3]. There are; however, different cutoff values across
ethnicities and gender. Currently, the existing cut-off
points for Thai people are in the domains of muscle
strength and physical performances [4, 5] but still lacking
in the domain of skeletal mass as well as the predictors of skeletal muscle mass. The AWGS recommends the
cutoff points to determine low skeletal muscle mass by
using 2 standard deviations (SD) below the mean relative
appendicular muscle mass (RASM) of a young reference
group 20-39 years or the lower quintile of the group, and
recommends using height-adjusted skeletal muscle mass
instead of weight-adjusted skeletal muscle mass [3].
Factors associated with low skeletal muscle mass of older
adults in previous reports are older age, being women,
being African-Americans compared to Caucasians,
physical disability, physical inactivity, smoking, living in
the city as compared to the rural area, lower testosterone
and vitamin D levels [2, 6-8]. For body mass index and fat
mass, the results are inconsistent as some reports showed
positive and some showed negative relationships [6, 9].
As sarcopenia increases with age and many factors related
to this condition are reversible and lack study in this area
of Thailand, recognizing those factors and their effects on
the size of skeletal muscle mass would benefit for early
intervention to young adults to delay the consequences
of sarcopenia. Thus, the primary objective of this study
was to identify risk factors of low skeletal muscle mass
in young adults by sex-specific groups, and the secondary
objective was to identify optimal cut-off points of low
skeletal muscle mass of young Thai adults by specific sex
groups. |
Methods |
| Study Subjects |
| This was a cross-sectional study. Subjects were a sample
of young healthy Thai adults who were aged 20-39 years.
The potential subjects were sent a letter of invitation asking
if they would like to participate in the study. The exclusion
criteria were the persons who could not stand upright, had
a pacemaker, used drugs, herbs or any hormones that affect
muscle mass and strength such as contraceptive drugs,
steroid hormones and thyroid hormone, drank alcohol 12
hours prior to the test, had vigorous exercise within 12
hour before analysis, were dehydrated, and women during
the menstrual period or pregnancy. The study population
was similar to the previously published article [10]. |
| Setting |
| The setting was Khon Kaen province, Thailand which is
the second-largest Northeastern province. It is located 445
km from Bangkok with a population of 1.8 million [6]. |
| Data Collection |
| Baseline data including age, educational level, smoking
status and level of physical activity using the General
Practice Physical Activity Questionnaire (GPPAQ) were
collected. GPPAQ is a validated screening tool and is
correlated to cardiovascular disease risks that provide a
simple, 4-level physical activity index (PAI) of; active,
moderately active, moderately inactive and inactive or
sedentary lifestyles [11]. Body weights and standing
heights were taken while subjects were lightly clothed and wore no shoes. Body composition including fat
mass (FM), percent body fat (PBF), free fat mass (FFM),
total body water (TBW) estimations were determined
using the Tanita bioelectrical impedance analysis (BIA)
system (model BC-418). Body mass index (BMI) was
calculated as weight (kg) divided by height squared (m2).
Appendicular skeletal mass (ASM) was determined from
the sum of predicted skeletal mass from arms and legs (kg)
and then the calculation of relative appendicular skeletal
mass (RASM) was derived from ASM (kg) divided by
height squared (m2) [3]. |
| Procedure |
| Eligible subjects had no food or drink 4 hours prior the
test and written informed consent was obtained from
all subjects. Baseline data collected by trained nurses
included age, sex, educational level, smoking status (never
smoked, ex-smoker and current smoker), level of physical
activity using GPPAQ, body weight, height, and then body
composition which included fat mass, percent body fat,
free fat mass, total body water, and appendicular skeletal
mass was measured by the body composition monitor with
a scale. |
| Sample Size Calculation |
| Sample size calculation was based on the primary
objective of this study which was based on the factors
associated with skeletal muscle mass. It was anticipated
that there would be 9 factors associated with ASM among
the subjects: age, gender, ethnicity, physical disability,
sedentary lifestyle, smoking status, living area (urban or
rural area), body mass index and fat mass [2, 6, 7, 12-
14]. The sample size estimation was based on the current
recommendation among statisticians for multiple logistic
regression analysis, i.e., that the number of subjects being
five to ten times the number of risk factors in the multiple
logistic model [15]. As gender is the strong factor associated
with the discrepancy in body composition in many studies,
analyzing the data as sex-specific was performed [6, 16].
Gender, ethnicity, living area and physical disability were
controlled by the study design; therefore, approximately
at least 60 subjects of each sex were needed. This study
recruited 100 men and 100 women. |
| Statistical Analysis |
| Demographic data were analyzed using descriptive
statistics, presentations in percentage, mean and standard
deviations. If the distribution of these data was not a
normal distribution, then medians, and inter-quartile
ranges were used instead. Factors associated with RASM
were analyzed using univariate and multiple logistic
regressions. Univariate analysis was used to examine
all associated factors. Multi-collinearity of those factors
was checked, then factors with P<0.20 were entered into
a multiple logistic regression model with logarithmic
transformations. P<0.05 was considered to indicate
statistically significant differences and adjusted odds ratios (OR) and their 95% confidence intervals (CI) were
reported to consider the strength of association between
possible factors and RASM. All the data analyses were
carried out using STATA version 10.0 (Stata Corp, College
Station, Texas). |
| Ethics approval was provided by the ethics committee of
the Faculty of Medicine, Khon Kaen University according
to the Helsinki Declaration. |
Results |
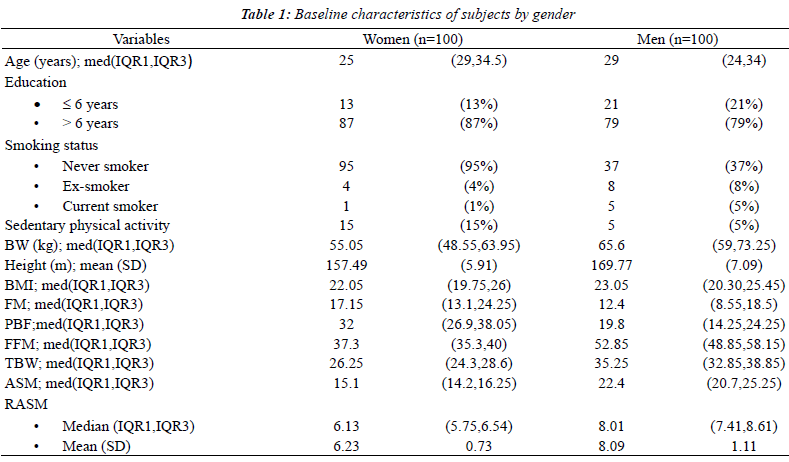
| One-hundred men and 100 women were recruited for this
study. Baseline characteristics and body compositions
are shown in Table 1. The cut-off points of the RASM
at the 20th percentile that were used to define having low muscle mass were; for women, 5.68 kg/m2 and for men,
7.22 kg/m2. |
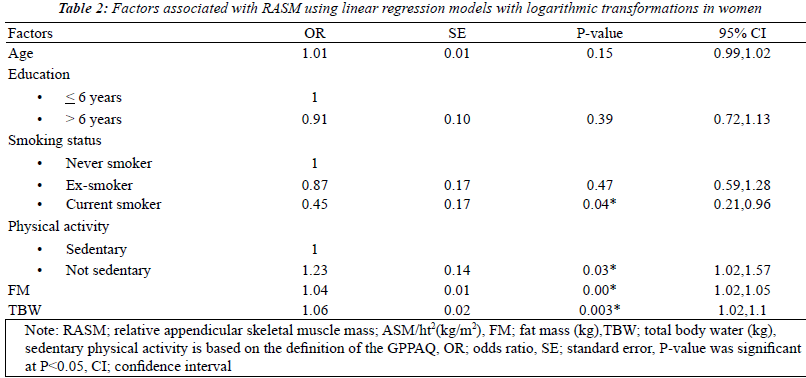
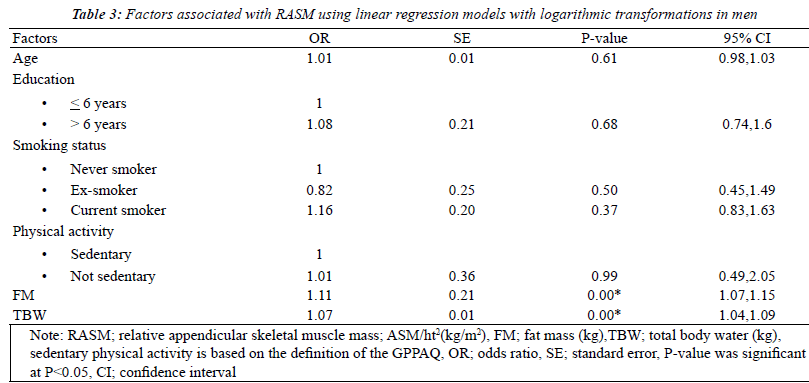
| The factors associated with RASM by gender using
univariate analysis models that were adjusted for the
baseline characteristics were fat mass and total body water.
Seven factors were found to be statistically significant
(p<0.2): age, levels of education, smoking status, physical
activity, BMI, FM, and TBW. After multicollinearity was
checked, BMI was removed from the model. With multiple
logistic regressions with logarithmic transformations, 4 of
these factors were found to be independently related to
RASM in women: smoking status, physical activity, fat
mass and total body water. For men, only 2 factors, FM
and TBW, showed statistical significance (Tables 2 and 3). |
 |
 |
 |
Discussion |
| This study represents the body compositions of young
Thai adults in northeastern part of Thailand where there
is evidence that the living area is one of the predictors
of skeletal muscle mass which was probably due to the
activity-related and nutritionally related causes [6]. There
is no cut-off value for skeletal muscle mass in young Thai
adults as a reference point for detection of low skeletal
muscle mass and the AWGS recommends using heightadjusted
skeletal muscle mass presented as RASM at
the lowest quintile as cut-off points. The optimal cutoff
points of low RASM in this study according to the
criteria of the AWGS for young Thai adults were 5.68 kg/
m2 in women and 7.22 kg/m2 in men. The cutoff values
determined in this study that are sex specific are close to
the references of the recommendation of the AWGS which
were 5.7 kg/m2 in women and 7.0 kg/m2 in men by using
BIA [3]. Therefore, Asian populations appear to have
similar standard muscle mass indices even with a diversity
of lifestyles and cultural backgrounds. |
| Factors related with RASM in this study for women were
being a current smoker, levels of physical activity, fat mass
and total body water. For men, only fat mass and total body
water showed the associations. Fat mass and TBW showed
positive associations with RASM in both sexes, supporting
the evidence that fat mass was a predictor of appendicular
muscle mass regardless of sex [9, 12, 16, 17]. The possible
explanation is likely the result that the increased muscle
mass is related to increased muscle hydration on account
of edema or intramuscular fat and probably fibrous tissue
deposition [16]. |
| For smoking, it is well-documented that smoking,
particularly in current smokers, is associated with low
RASM. The potential mechanism is directly from an
impairment of muscle protein synthesis and up-regulating genes associated with muscle maintenance [14]. Poor
lifestyle habits such as a sedentary lifestyle and poor
nutrition are indirect mechanisms of smoking [14]. This
study demonstrated this effect only in women who are
current smokers though there was only one currently
smoking participant. Hormonal differences by gender may
play an important role, especially testosterone, which has
positive effects on skeletal muscle mass [8]. In addition,
previous studies demonstrated the effect of smoking
on muscle mass in older adults whereas this study was
conducted in young adults. Therefore, this study could
not show the effect of smoking on muscle mass in young
men [14, 18-20]. The results of this study also confirm the
effect of a sedentary lifestyle on muscle mass in females.
Physical inactivity causes an imbalance of protein
synthesis and breakdown. The activation of different
proteolytic systems appears to activate muscle cell
damage [14]. For men, there was no statistical difference
attributable to sedentary lifestyle. This can be explained by
the comparable reasons described for smoking, hormonal
differences and age effects [18, 19]. Age showed no
association with low muscle mass in young Thai adults
after controlling for other variables whereas it is one of the
significant factors among older adults in many studies [7,
8, 12]. This indicates that muscle damage that is related to
low skeletal muscle mass index does not occur before the
age of 40, supporting the prior reviews [1, 2]. |
| The limitations of this study were the factors that might
be associated with skeletal mass in young adults such as
testosterone levels that were not measured. In addition, the
results might not be generalized to different populations. |
| In conclusion, the results from this study confirm the
standard cutoff values of low RASM among Asians as
recommended by the AWGS. Age-related changes in
muscle do not appear to effect skeletal muscle mass in
young adults. Fat mass and total body water are positively associated with RASM independent of gender whereas
lifestyle habits including current smoking and sedentary
lifestyle are predictors for low ASM in young Thai
women. This study emphasizes the importance of lifestyle
modification in young adults, even in men, because
there is no harm done by improving lifestyle. The cost
effectiveness would be superior to the cost of treatments
when adverse outcomes of low ASM exist. |
Acknowledgments |
| We wish to acknowledge Professor James A. Will,
University of Wisconsin-Madison, for editing the
manuscript via the Faculty of Medicine Publication Clinic,
Khon Kaen University, Thailand. |
Funding |
| This manuscript was funded by the Neuroscience Research
and Development Group, Khon Kaen University, Thailand
under grant number 001/2557 and the Thailand Research
Fund (number IRG 5780016). |
Disclosures |
| The authors declared no potential conflicts of interest with
respect to the authorship and/or publication of this article. |
References |
- Kim TN, Choi KM. Sarcopenia: Definition, Epidemiology, and Pathophysiology. J Bone Metab 2013;20:1-10.
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am GeriatrSoc 2002;50:889-896.
- Chen LK, Liu LK, Woo J, AssantachaiP, Auyeung TW, Bahyah KS. Sarcopenia in Asia: consensus report of the asian working group for sarcopenia. J Am Med DirAssoc 2013;15:95-101.
- Assantachai P, Muangpaisan W, Intalapaporn S, Sitthichai K, Udompunturak S. Cut-off points of quadriceps strength, declines and relationships of sarcopenia-related variables among Thai community-dwelling older adults. GeriatrGerontolInt 2014;14:61-68.
- Praditsuwan R, Dajpratham P, Kuptniratsaikul V. The Timed Up & Go: a practical basic mobility skills assessment in the elderly. Siriraj Med J 2006;58:588-591.
- Pongchaiyakul C, Limpawattana P, Kotruchin P, Rajatanavin R. Prevalence of sarcopenia and associated factors among Thai population. J Bone Miner Metab 2013;31:346-350.
- Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J ApplPhysiol 1997;83:229-239.
- Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev 1999;107:123-136.
- Newman AB, Kupelian V, Visser M, Simonsick E,Goodpaster B, Nevitt M. Sarcopenia: alternative definitions and associations with lower extremity function. J Am GeriatrSoc 2003;51:1602-1609.
- Limpawattana P, Kengkijkosol T, Assantachai P, Krairit O, Pimporm J. The performance of obesity screening tools among young Thai adults. J Community Health 2014;39:1216-1221.
- Physical activity policy, health improvement directorate. General practise physical activity questionnaire. London: National Health Service; 2009.
- Janssen I. The epidemiology of sarcopenia.ClinGeriatr Med 2011;27:355-363.
- Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J ClinNutr 2002;76:473-481.
- Rom O, Kaisari S, Aizenbud D, Reznick AZ. Lifestyle and sarcopenia-etiology, prevention, and treatment. Rambam Maimonides Med J 2012;3:e0024.
- Katz MH. Multivariable Analysis: a Practical Guide for Clinicians. 2nd ed Cambridge: Cambridge University Press; 1999.
- Proctor DN, O'Brien PC, Atkinson EJ, Nair KS. Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. Am J Physiol 1999; 277:E489-495.
- Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J ApplPhysiol (1985) 2000;89:465-471.
- Castillo EM, Goodman-Gruen D, Kritz-Silverstein D, Morton DJ, Wingard DL, Barrett-Connor E. Sarcopenia in elderly men and women: the Rancho Bernardo study. Am J Prev Med 2003;25:226-231.
- Szulc P, Duboeuf F, Marchand F, Delmas PD. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J ClinNutr 2004;80:496-503.
- Petersen AM, Magkos F, Atherton P, Selby A, Smith K, Rennie MJ. Smoking impairs muscle protein synthesis and increases the expression of myostatin and MAFbx in muscle. Am J PhysiolEndocrinolMetab 2007;293:E843-848.
|


