ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 2
The predictive value of signal peptide-CUB-EGF domain-containing protein1 (SCUBE1) in terms of the duration of peripheral ischemia and exposure to reperfusion
Ozgur Sogut1, Ertan Sönmez2, Mehmet Yigit1, Kenan Ahmet Turkdogan3*, Okkes Taha Kucukdagl?4, Eda Yigit5, Omer Faruk Ozer6 and Cemil Civelek7
1Department of Emergency Medicine, Haseki Training and Research Hospital, Istanbul, Turkey
2Department of Emergency Medicine, Faculty of Medicine, Bezmialem Vakif University, Istanbul, Turkey
3Department of Emergency Medicine, Faculty of Medicine, Adnan Menderes University, Ayd?n, Turkey
4Department of Emergency Medicine, Bak?rköy Sadi Konuk Training and Research Hospital, Istanbul, Turkey
5Department of Emergency Medicine, Sisli Etfal Training and Research Hospital, Istanbul, Turkey
6Department of Biochemistry, Faculty of Medicine, Bezmialem Vakif University, Istanbul, Turkey
7Department of Emergency Medicine, Kanuni Sultan Suleyman Training and Research Hospital, Istanbul, Turkey
- *Corresponding Author:
- Kenan Ahmet Turkdogan
Department of Emergency Medicine
Faculty of Medicine
Adnan Menderes University
Ayd?n, Turkey
Accepted on April 29, 2016
Biomarkers play important roles in the diagnosis and follow-up of ischemic diseases. This study investigated the predictive value of signal peptide-CUB-EGF domain-containing protein1 (SCUBE1) in rats with peripheral ischemia that were subjected to reperfusion. Forty rats were divided into five equal groups as follows (eight rats per group): a control group (to determine the basal levels of the markers), three ischemia groups (groups 1-3), and a reperfusion group (group 4). There were no significant differences in SCUBE1 levels between the control and ischemia groups. However, the SCUBE1 level was markedly higher in the reperfusion group than in the control and ischemia groups (p<0.05). The results indicate that SCUBE1 levels are not biomarkers of the induction of ischemia. However, they are potentially markers of reperfusion in rats with acute peripheral occlusions.
Keywords
Signal peptide-CUB-EGF domain-containing protein 1, Peripheral ischemia, Reperfusion.
Introduction
Ischemic exposure is an important determinant of tissue recovery, which in turn affects cellular metabolism. Critical limb ischemia is a frequent ischemic disorder, leading to amputations of the extremities if normal blood flow does not promptly resume [1,2]. High amputation (30%) and mortality rates (25%), together with unresolved pain and tissue loss (20%), have been reported in the literature [1]. Therefore, recent studies have focused on the identification of novel markers of the duration of ischemia and reperfusion in models of critical limb ischemia [1,3].
Signal peptide-CUB-EGF domain-containing protein1 (SCUBE1) is a surface membrane glycoprotein of human platelets and endothelial cells [4,5]. The levels of SCUBE1 have been reported to be higher in platelets than endothelial cells [5]. The α granules of inactive platelets store the protein. Upon activation by thrombin, the granules are translocated to the surface of platelets, proteolytically released, and incorporated into a thrombus as smaller soluble fragments [5]. SCUBE1 is released by endothelial cells in response to ischemic injury [6,7], and platelet activation and aggregation may be responsible for ischemic complications in patients with acute coronary syndrome and acute ischemic stroke [8]. SCUBE1 levels measured within 6 h after onset of ischemic symptoms have the potential to serve as an early-stage damage marker in patients with acute thrombotic diseases [8]. The roles played by SCUBE1 under ischemic conditions and during reperfusion demonstrate that SCUBE1 measurement can be useful when examining endothelial responses during hypoxia or after blood flow recovery.
In this experimental study, plasma SCUBE1 levels were investigated during peripheral ischemia and after reperfusion.
Material and Methods
All procedures complied with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals. Before the study commenced, all procedures were checked and approved by the local animal ethical committee.
Animals
Forty male albino Wistar rats weighing between 250 g and 275 g and aged 12-14 weeks were included. All rats were obtained from the Laboratory Animal Production Unit of our university. Prior to the study, the animals were maintained in standardized cages under constant humidity (50 ± 5%) and temperature (22 ± 2°C) with a 12 h/12 h light/dark cycle.
Study design and stages
Five groups were created, with eight rats in each group. All rats were anesthetised with ketamine (Ketalar, Pfizer) at a dose of 130 mg/kg and xylasine (Rompun, Bayer) at a dose of 20 mg/kg via an intraperitoneal catheter. Anesthesia was maintained with ketamine hydrochloride (50 mg/kg). The ischemia/reperfusion model was similar to those previously described by Saray et al. [9] and Yavuz et al. [3] and featured 8 h of ischemia followed by 60 min of reperfusion upon clamping of the femoral artery. In all groups, the rats were sacrificed immediately after blood samples were taken.
The procedures performed on each group were as follows:
Control group (n=8): Intracardiac blood samples were obtained to determine the baseline values of the biomarkers; we did not perform any intervention.
Group I–III (n=8): Peripheral ischemia was created by clamping the right femoral artery via a simple femoral incision. Intracardiac blood samples were obtained 2, 4, and 8 h later.
Group IV (n=8): Peripheral ischemia was created for 8 h by clamping the right femoral artery via a simple femoral incision. After 8 h of ischemia, reperfusion was induced for 1 h, and intracardiac blood samples were obtained during this first hour of reperfusion.
SCUBE1 measurement
Plasma SCUBE1 levels were assayed using a commercially available enzyme-linked immunosorbent assay kit (Catalog No. CSBE15005 h, Cusabio Biotech Co., Wuhan, Hubei, P.R. China). The results are expressed in ng/mL (0.16 ng/mL was the minimum detectable level) as described by Turkmen et al. [10].
Statistical analysis
All analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL). Continuous variables are expressed as means ± standard deviations or as medians with the 25th–75th percentile ranges (when the parameters were normally and not normally distributed, respectively). In each group, follow-up comparisons (control; and 2, 4, and 8 h after reperfusion) were performed using Friedman’s two-way analysis. A two-tailed p<0.05 was considered significant.
Results
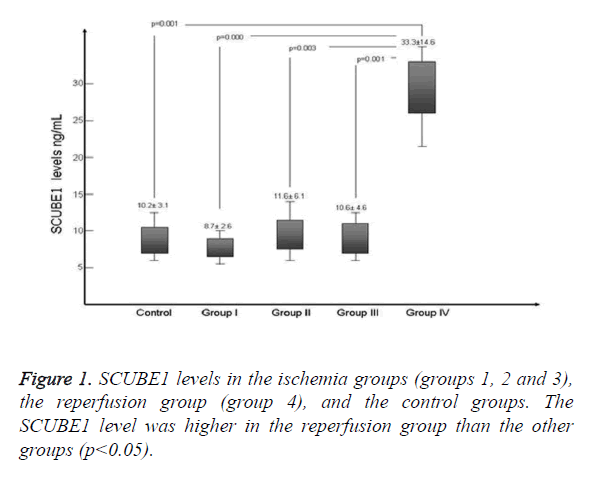
In the ischemia period, basal SCUBE1 levels did not significantly increase (p>0.05). However, the levels of the reperfusion group were significantly elevated compared to those of the control and ischemia groups (p<0.05). Figure 1 compares the SCUBE1 levels of the groups.
Discussion
SCUBE1 is a novel biomarker secreted by platelets and endothelial cells, and has been studied in the context of various types of tissue ischemia and reperfusion injury [9,10]. These studies have demonstrated that SCUBE1 is highly expressed in various developing tissues (gonads, the nervous system, somites, the surface ectoderm, and limb buds) of mouse embryos and that the protein is secreted from human vascular endothelium at various sites around the body [4,6,11]. SCUBE1 levels in hypertensive crises [12] and in patients with acute coronary syndrome have been investigated [8,13]. Turkmen et al. [10] reported that SCUBE1 levels were elevated in an experimental mesenteric ischemia model. It was concluded that enhanced platelet activation might contribute to the rise in SCUBE1 levels noted in mesenteric ischemia. However, we did not detect any significant elevation in SCUBE1 levels during 8 h of peripheral ischemia. On the other hand, the SCUBE1 level was clearly elevated in the reperfusion group. Zhuang et al. [7] reported that SCUBE1 played an important role after renal ischemia-reperfusion injury. It was claimed that SCUBE1 might be involved in regeneration, combined with regulation of tubular cell proliferation and re-epithelialization of damaged kidneys following ischemia-reperfusion injury. We suggest that an elevated SCUBE1 level may be an indicator of platelet activation and may also predict endogenous remodeling.
Conclusion
The SCUBE1 level was elevated only in the reperfusion group, in contrast to the control and ischemia groups. We suggest that the SCUBE1 level can be used to show that reperfusion is in play. Evaluation of the SCUBE1 level may also be helpful during follow-up of re-canalization and re-occlusion. However, further studies on SCUBE1 levels in re-occlusion models are needed.
Limitations
SCUBE1 is a novel biomarker and the SCUBE1 level may vary significantly in other ischemia-reperfusion models including renal, liver, or gastrointestinal ischemia. We have not yet used other animal models to address this issue. Another limitation is that we did not measure the levels of mRNA encoding SCUBE-1 in the peripheral vascular tissue of rats exposed to ischemia and reperfusion. In addition, we did not determine whether peripheral SCUBE-1 injection could alleviate ischemia or reperfusion injury in rats. Thus, this is a preliminary study of the utility of SCUBE1 as a biomarker of ischemia-reperfusion injury induced by clamping the femoral artery. More work is needed to validate our results.
References
- Feiring AJ, Krahn M, Nelson L, Wesolowski A, Eastwood D, Szabo A. Preventing leg amputations in critical limb ischemia with below-the-knee drug-eluting stents: the PaRADISE (PReventing Amputations using Drug eluting StEnts) trial. J Am CollCardiol 2010; 55: 1580-1589.
- Yavuz C, Yazici S, Karahan O, Demirtas S, Caliskan A, Guclu O, Ertas F, Mavitas B. Serum nitric oxide level could be a predictive biomarker for detection of critical ischaemia duration. Biomarkers 2013; 18: 116-120.
- Yavuz C, Caliskan A, Yazici S, Karahan O, Guclu O, Demirtas S. Human ischemia modified albumin can be a predictive biomarker for the detection of peripheral ischemia duration HealthMed 2012; 6: 3478-3481.
- Yang RB, Ng CK, Wasserman SM, Colman SD, Shenoy S, Mehraban F, Komuves LG, Tomlinson JE, Topper JN. Identification of a novel family of cell-surface proteins expressed in human vascular endothelium. J BiolChem 2002; 277: 46364-46373.
- Tu CF, Su YH, Huang YN, Tsai MT, Li LT, Chen YL, Cheng CJ, Dai DF, Yang RB. Localization and characterization of a novel secreted protein SCUBE1 in human platelets. Cardiovasc Res 2006; 71: 486-495.
- Favre CJ, Mancuso M, Maas K, McLean JW, Baluk P, McDonald DM. Expression of genes involved in vascular development and angiogenesis in endothelial cells of adult lung. Am J Physiol Heart CircPhysiol 2003; 285 :H1917-1938.
- Zhuang J, Deane JA, Yang RB, Li J, Ricardo SD. SCUBE1, a novel developmental gene involved in renal regeneration and repair. Nephrol Dial Transplant 2010; 25: 1421-1428.
- Dai DF, Thajeb P, Tu CF, Chiang FT, Chen CH, Yang RB, Chen JJ. Plasma concentration of SCUBE1, a novel platelet protein, is elevated in patients with acute coronary syndrome and ischemic stroke. J Am CollCardiol 2008; 51: 2173-2180.
- Saray A, Can B, Akbiyik F, Askar I. Ischaemia-reperfusion injury of the peripheral nerve: An experimental study. Microsurgery 1999; 19: 374-380.
- Turkmen S, Mentese S, Mentese A, Sumer AU, Saglam K, Yulug E, Turedi S, Gunduz A. The value of signal peptide-CUB-EGF domain-containing protein 1 and oxidative stress parameters in the diagnosis of acute mesenteric ischemia. AcadEmerg Med 2013; 20: 257-264.
- Grimmond S, Larder R, Van Hateren N, Siggers P, Hulsebos TJ, Arkell R, Greenfield A. Cloning, mapping, and expression analysis of a gene encoding a novel mammalian EGF-related protein (SCUBE1). Genomics 2000; 70: 74-81.
- Karabacak M, Yi?it M, Turkdogan KA, Yi?it E, Selek S. Is signal peptide-CUB-EGF domain-containing protein1 a diagnostic biomarker in patients with hypertensive crises. ClinHemorheolMicrocirc 2016; 61: 513-522.
- Sonmez E, Turkdogan KA, Karabacak M, Civelek C, Yilmaz C, Ozer OF, Çavu? UY. The diagnostic role of signal peptide-C1r/C1s, Uegf, and Bmp1-epidermal growth factor domain-containing protein 1 in non-ST-elevation acute coronary syndrome. Am J Emerg Med 2015; 33: 21-24.