ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 5
The expressions and roles of S100A6 and S100A10 in gastric cancer
1Department of Gastroenterology and Hepatology, The 309 Hospital of PLA, PR China
2Department of Pediatrics, the First Affiliated Hospital of Hebei Northern University, PR China
#These authors contributed work equally for this research
The First Affiliated Hospital of Hebei Northern University and the 309 Hospital of PLA contributed equally in the present research.
- *Corresponding Author:
- Lin Zhang
Department of Gastroenterology and Hepatology
The 309 Hospital of PLA Beijing, PR China
Accepted on October 13, 2016
Aim: S100A6 and S100A10 genes are upregulated in many human malignant tumors. But, little is known about their expressions or roles in gastric adenocarcinoma. In the present study, we intended to investigate the expressions and clinicopathologic significance of the two genes in gastric adenocarcinoma.
Methods: The immunohistochemical assays were performed to detect the expressions of S100A6 and S100A10 in 62 specimens and their correlation with clinicopathologic features was examined.
Results: In all 62 gastric adenocarcinoma specimens, 27 (43.5%) and 20 (32.3%) had a strong expression of S100A6 and S100A10. In the adenocarcinoma specimens with a strong S100A6 expression, the proportion of poorly differentiated adenocarcinomas was significantly higher than that in specimens with a weak S100A6 expression (P<0.05). The proportions of lymph node metastasis and the depth of the tumors in the specimens with a strong S100A6 or S100A10 expression were higher significantly too. The results of survival analysis showed that the prognosis of patients whose specimens had a strong S100A6 expression was poorer than that of patients with a weak expression significantly (P<0.05).
Conclusions: The expressions of S100A6 and S100A10 could be correlated with poor differentiation and metastasis of gastric adenocarcinoma. In some distance, S100A6 may be considered a potential marker for gastric adenocarcinoma with prognostic value.
Keywords
Gastric cancer, S100A6 gene, S100A10 gene, Immunohistochemistry, Prognosis.
Introduction
Gastric adenocarcinoma is most common malignant tumor and considered one of the most lethal forms of cancer because of the difficulties in diagnosis early and therapy effectively [1,2]. In China, the patients with gastric adenocarcinoma often have poorer prognosis than the patients in western countries. Recently, it have been reported that more and more genes and their products could have some important roles in gastric carcinogenesis as a complex multifactorial process [3-5].
In our results of the comparative proteome analysis [6], it was showed that the expressions of S100A6 and S100A10 were upregulated remarkably in a form of precancerous lesion (varioliform gastritis) of gastric cancer when compared with the peripheral normal gastric mucous membrane. Thus, we considered that such upregulated expressions of S100A6 and S100A10 could be consistent with the greater proliferation, as well as enhanced invasive activity, would be likely to potential oncogene. S100A6 and S100A10 are important members of S100 family. The genes of this family are distributed in the region 1q21-1q22. There are about 20 proteins that contain well-conserved EF-hand calcium binding domains in S100 protein family [7-11]. The first member of this protein family was isolated from bovine brain tissue and was called bovine brain S100 protein because of its solubility in a 100% saturated solution of ammonium sulphate [12]. The S100 proteins, with molecular weights between 9 and 14 kDa, have been shown to interact with transcriptional factors and may be involved in the regulation of protein phosphorylation, calcium homeostasis, cell proliferation, and differentiation [7,9,10]. They also could interact with the cytoskeleton and may thereby influence cell motility [9,10,13-16]. Several S100 proteins have been associated with human cancers such as S100A2, S100A4 and S100A6 [17-20]. S100A6 (Calcyclin) is an EF-hand calcium binding protein belonging to the S100 family. S100A6 cDNA was discovered by Hirschhorn et al. in 1984 and further characterized by Calabretta et al. in1986 [21]. The properties and distribution of S100A6 are well established but its function is unclear. It has been suggested that S100A6 might be involved in cell proliferation, differentiation, and secretion. In recent years, the upregulation of S100A6 has been reported in a variety of tumors such as colorectal carcinoma, pancreatic carcinoma, hepatic carcinoma, cholangiocarcinoma, sweat gland tumors, and papillary thyroid carcinoma et al. and linked to metastasis [22-28].
However, exact intracellular roles of S100A6 related with cancer have not been clarified yet. Yang et al. performed the serial analysis of gene expression (SAGE) experiment, microarrays and real-time RT-PCR assays to compare the gene expression profiles between cancerous and adjacent tissues [29]. They found that S100A6 was significantly upregulated in gastric cancer tissue and thought that it was associated with gastric cancer tumorigenesis and quantitation of S100A6 is a promising tool for diagnosis of gastric cancer. On the other hand, Ning X et al. prepared CacyBP/SIP (Calcyclin -binding protein which could interact with S100A6) overexpressing and knockdown cell lines of gastric cancer [30]. They found that CacyBP/SIP could inhibit the proliferation of gastric cancer cells, suppressed tumorigenicity in vitro, and prolonged the survival time of tumor-bearing nude mice. So, their results suggested that CacyBP/SIP could be a potential inhibitor of cell growth and invasion in the gastric cancer cell and it could be an antagonist to the role of S100A6 in gastric cancer. But, at present, the reports on the relationship between S100A6 gene and the clinicopathologic features of the patients with gastric cancer were very limited. S100A10 protein, namely p11, is an important member of the S100 family of proteins containing two EF-hand calcium-binding motifs. This protein is localized in the cytoplasm and/or nucleus of a wide range of cells. S100A10 is linked with the transport of neurotransmitters.
It was reported that S100A10 expressed in the brain of humans and it has an important role in the regulation of mood. S100A10 protein interacts with plasma membrane proteins through its association with annexin II as an integral part of cellular structural scaffolding.
At present, more and more reports show that the expression of S100A10 could upregulate in many human cancer, such as ependymoma, breast cancer, renal cell carcinoma and lymphoma [31-34]. El-Rifai et al. demonstrated the overexpression of S100A10 in gastric cancer using serial analysis of gene expression (SAGE) [35]. Tsai et al. reported that S100A10 was down-regulated in the patients with gastric preneoplasia receiving H. pylori eradication therapy but upregulated in the placebo group [36]. These results could suggest that altered expression of S100A10 might be associated with the oncogenesis of gastric cancer. On the other hand, the report on the clinicopathologic significance of S100A10 expression in gastric adenocarcinoma was very limited.
To investigate the expressions and the roles of S100A6 and S100A10, we examined their immunohistochemical expressions in gastric cancer and elucidated the further clinicopathologic significance of the two genes in the current study.
Material and Methods
Materials
S100A6 monoclonal antibody was purchased from Abcam company (USA), S100A10 polyclonal antibody was purchased from ProteinTech Group, inc (USA). Rabbit antimouse HPR (1:1,000; Dako, Cophenhagen, Denmark) was used to recognize S100A6 antibody and goat antirabbit HPR (1:1,000; Biosynthesis Co., LTD, China) was used to recognize S100A10 antibody.
Sample collection
Samples were taken from 62 patients with gastric cancer in the 309 Hospital of PLA from 2005 to2010. These patients were diagnosed by gastroscope examination and biopsy, then the results wer confirmed in operation of gastric resection, 3-field lymph node dissection and reconstruction of the digestive tract. They had not been treated with any preoperative therapy. All samples were gotten in operation for these patients. From every patient, four pieces of tissue of the tumors and normal mucosa were gotten respectively and the tissue was embedded in paraffin for future use. They were classified as well and poorly differentiated according to the pathological diagnosis. Of the 62 patients, 39 were men, and 23 were women, the mean age was 62.4 years (62.4 ± 9.6). The patients were all well informed in accordance with the disciplines of the Ethics Committee of Biomedicine, The 309 Hospital of PLA, China. The clinicopathologic stages were assessed according to the tumor-node-metastasis classification of malignant tumors described by the International Union against Cancer [37]. Follow-up for these patients was performed from operation to death. The interval of the follow-up period ranged from 6 months to 73 months and the mean value was 28 months and 4 days.
Immunohistochemical assays of S100A6 and S100A10
Tissue sections of 5 μm thickness were cut from paraffinembedded tissue blocks, placed on object slides precoated with silane, and incubated for 20 minutes in a thermostat at 60°C. After washing in xylene and a graded series of ethanol for removing the paraffin, the sections were immersed in phosphate buffered saline (PBS) for 10 minutes. They were treated with 2% bovine serum albumin (BSA) to block nonspecific staining, then with 0.1% Triton X-100 in PBS for1h at room temperature. After treated with 3% H2O2 for 10 min to block endogenous peroxidase activity. After wasing in PBS for 10 minutes, monoclonal anti-S100A6 antibody and polyclonal anti-S100A10 antibody were applied for 1 h respectively, the secondary antibody–biotin labelled rabbit antimouse and antigoat HPR were added respectively for 20 minutes after removing primary antibody by washing in PBS thrice. The sections were washed in PBS for 10 minutes, then 3,3’- diaminobenzidine (DAB) was used as the chromogen. Finally, the sections were counterstained with Harris’s haematoxylin for 3 min and coverslips applied with a xylene-based mounting medium. For the semiquantifications of S100A6 and S100A10 immunostaining were analyzed based on the criteria that were presented by Kase et al. [38] and Tadahiro et al. [39]. The S100A6 or S100A10 positive proportion was expressed as the percentage of the number of S100A6 or S100A10 labelled cell divided by the total number of cell examined under a microscope (20· objective) and the average value of 10 fields was calculated. A strong expression was determined when more than 50% of the carcinoma cells in a specimen showed positive signals. Otherwise those with less than 50% of the carcinoma cells showing positive signals were considered to have a weak expression.
Statistical analysis
The Student t test and chi-square test were used to compare the data. Survival analysis was performed using the Life-Table method and Spearman’s correlation test was used for the detection of any correlation between the expressions of S100A6 and S100A10. The criterion for statistical significance was P<0.05.
Results
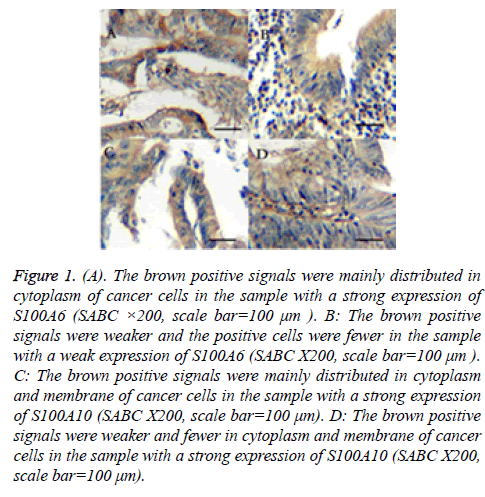
The results of immunohistochemical assay of S100A6 showed that the brown positive signals were mainly distributed in cytoplasm of cancer cells and there were a few positive signals in cell nucleus. That of S100A10 showed that the positive signals were mainly distributed in cytoplasm and membrane (Figure 1). The results of statistical analysis of relationship between the two genes and clinicopathologic characteristics were shown in Tables 1 and 2. Fifty-six in all sixty-two had an expression of S100A6 (positivity rate: 90.3%). In all positive samples, twenty-seven (48.2%) had a strong expression of S100A6, otherwise other twenty-nine (51.8%) had a weak one. On the other hand, fifty in all sixty-two had an expression of S100A10 (positivity rate: 80.6%). In positive samples, nineteen (38.0%) had a strong expression of S100A10, otherwise other thirty-one (62.0%) did not. There was no significant difference between the patients with a strong expression of S100A6 or S100A10 and those with a weak expression regard to sex, age or the location of tumors. On the other hand, the proportion of poorly differentiated adenocarcinomas was significantly higher in specimens with a strong S100A6 expression than that in specimens with a weak expression (P<0.05). The proportion of lymph node metastasis and the depth of the tumors in the specimens with a strong S100A6 or S100A10 expression were higher significantly too (P<0.05). To sum up, the stage of the tumors were significantly different between the two groups.
Figure 1: (A). The brown positive signals were mainly distributed in cytoplasm of cancer cells in the sample with a strong expression of S100A6 (SABC ×200, scale bar=100 μm ). B: The brown positive signals were weaker and the positive cells were fewer in the sample with a weak expression of S100A6 (SABC X200, scale bar=100 μm ). C: The brown positive signals were mainly distributed in cytoplasm and membrane of cancer cells in the sample with a strong expression of S100A10 (SABC X200, scale bar=100 μm). D: The brown positive signals were weaker and fewer in cytoplasm and membrane of cancer cells in the sample with a strong expression of S100A10 (SABC X200, scale bar=100 μm).
| Weak expression of S100A6 |
Strong expression of S100A6 |
Statistical significance |
|
|---|---|---|---|
| Age (years) | 61.3 ± 9.7 | 62.6 ± 11.3 | P>0.05 |
| Sex | |||
| Male | 18 (62.1%) | 17 (63.0%) | P>0.05 |
| Female | 11 (37.9%) | 10 (37.0%) | P>0.05 |
| Body weight(Kg) | 63±11.6 | 61±15.3 | P>0.05 |
| Height(m) | 1.66±0.34 | 1.69±0.26 | P>0.05 |
| Primary tumor diameter (cm) |
4.5±2.6 | 5.2±1.8 | P>0.05 |
| Depth of invasion of primary tumor | |||
| T0 | 0 | 0 | P>0.05 |
| T1 | 2(6.9%) | 3(11.1%) | P>0.05 |
| T2 | 7 (24.1%) | 6(22.2%) | P>0.05 |
| T3 | 13(44.8%)* | 7(25.9%) | P>0.05 |
| T4 | 7(24.1%) | 11(40.7%)* | P>0.05 |
| Location of the primary tumor | |||
| Cardia | 6 (20.7%) | 5(18.5%) | P>0.05 |
| Gastric body | 5(17.2%) | 4(14.8%) | P>0.05 |
| Gastric antrum | 12(41.4%) | 10(37.0%) | P>0.05 |
| Pylorus | 6(20.7%) | 8(29.6%) | P>0.05 |
| Lymph node metastasis |
11 (37.9%) | 16 (59.3%)* | P>0.05 |
| Vascular invasion | 9 (31.0%) | 13 (48.2%) | P<0.05 |
| Pathological type | |||
| Well differentiated adenocarcinoma |
8 (27.6%) | 4 (14.8%) | P<0.05 |
| Poor differentiated adenocarcinoma |
11 (37.9%) | 13 (48.1%)* | P<0.05 |
| Signet ring cell carcinoma |
5 (17.2%) | 6 (22.2%) | P>0.05 |
| Mucinous adenocarcinoma |
3 (10.3%) | 2 (7.4%) | P>0.05 |
| Undifferentiated carcinoma |
2 (6.9%) | 2 (7.4%) | P>0.05 |
Table 1. The relationship between the expression of S100A6 and clinicopathologic characteristics. Note: Qualitative data were percentages and quantitative data were expressed as mean±SD in this table, * denoted a significantly higher percentage.
| Strong expression of S100A10 | Weak expression of S100A10 | Statistical significance | |
|---|---|---|---|
| Age (years) | 60.7±11.4 | 63.2±13.5 | P>0.05 |
| Sex | |||
| Male | 12 (63.2%) | 18 (58.1%) | P>0.05 |
| Female | 7 (36.8%) | 13 (41.9%) | P>0.05 |
| Body weight(Kg) | 65.5±10.7 | 62.7±14.4 | P>0.05 |
| Height (m) | 1.64±0.41 | 1.65±0.0.33 | P>0.05 |
| Primary tumor diameter (cm) | 5.1±1.7 | 5.7±2.0 | P>0.05 |
| Depth of invasion of primary tumor | |||
| T0 | 0 | 0 | P>0.05 |
| T1 | 2 (10.5%) | 2 (6.5%) | P>0.05 |
| T2 | 2 (10.5%) | 8 (25.8%)* | P<0.05 |
| T3 | 8 (42.1%) | 14 (45.1%) | P>0.05 |
| T4 | 7 (36.8%)* | 7 (22.6%) | P<0.05 |
| Location of the primary tumor | |||
| Cardia | 4 ( (21.1%) | 6 (19.4%) | P>0.05 |
| Gastric body | 4 (21.1%) | 5 (16.1%) | P>0.05 |
| Gastric antrum | 9 (47.4%) | 15 (48.4%) | P>0.05 |
| Pylorus | 2 (10.5%)* | 5 (16.1%) | P>0.05 |
| Lymph node metastasis | 10 (52.6%)* | 12 (38.7%) | P<0.05 |
| Vascular invasion | 6 (31.6%) | 11 (35.5%) | P>0.05 |
| Pathological type | |||
| Well differentiated adenocarcinoma | 5 (26.3%) | 9 (29.0%) | P>0.05 |
| Poor differentiated adenocarcinoma | 9 (47.4%) | 13 (41.9%) | P>0.05 |
| Signet ring cell carcinoma | 2 (10.5%) | 4 (12.9%) | P>0.05 |
| Mucinous adenocarcinoma | 2 (10.5%) | 3 (9.7%) | P>0.05 |
| Undifferentiated carcinoma | 1 (5.3%) | 2 (6.5%) | P>0.05 |
Table 2. The relationship between the expression of S100A10 and clinicopathologic characteristics. Note: Qualitative data were percentages and quantitative data were expressed as mean ± SD in this table, *denoted a significantly higher percentage.
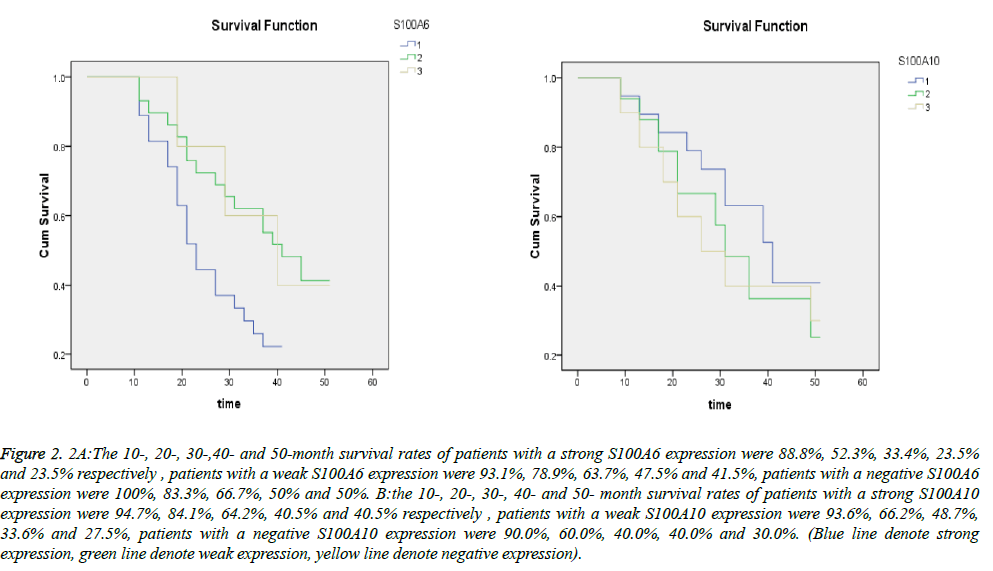
The results of survival analysis were shown as survival curves in Figure 2. The 10-, 20-, 30-,40- and 50-month survival rates of patients with a strong S100A6 expression were 88.8%, 52.3%, 33.4%, 23.5% and 23.5% respectively and those of patients with a weak S100A6 expression were 93.1%, 78.9%, 63.7%, 47.5% and 41.5%. The median survival time for the group with a strong S100A6 expression was 23.02 and that of the group with a weak expression was 41.14. In a word, The prognosis of patients with a strong S100A6 expression were significantly poorer than those with a weak expression (P=0.030<0.05). The survival curves showed that the 10-, 20-, 30-, 40- and 50- month survival rates of patients with a strong S100A10 expression were 94.7%, 84.1%, 64.2%, 40.5% and 40.5% respectively and those of patients with a weak S100A10 expression were 93.6%, 66.2%, 48.7%, 33.6% and 27.5%. The median survival time for the two groups was 34.82 and 29.25 respectively. The prognosis of patients with a strong S100A10 expression were not significantly poorer than those with a weak expression (P=0.333>0.05). S100A6 positivity rate of the S100A10-positive samples was 56% and S100A6 negativity rates of the S100A10-negative samples were 100%. However, the correlation between S100A6 and S100A10 expression in gastric cancer was not statistically significant (P>0.05).
Figure 2: 2A:The 10-, 20-, 30-,40- and 50-month survival rates of patients with a strong S100A6 expression were 88.8%, 52.3%, 33.4%, 23.5% and 23.5% respectively , patients with a weak S100A6 expression were 93.1%, 78.9%, 63.7%, 47.5% and 41.5%, patients with a negative S100A6 expression were 100%, 83.3%, 66.7%, 50% and 50%. B:the 10-, 20-, 30-, 40- and 50- month survival rates of patients with a strong S100A10 expression were 94.7%, 84.1%, 64.2%, 40.5% and 40.5% respectively , patients with a weak S100A10 expression were 93.6%, 66.2%, 48.7%, 33.6% and 27.5%, patients with a negative S100A10 expression were 90.0%, 60.0%, 40.0%, 40.0% and 30.0%. (Blue line denote strong expression, green line denote weak expression, yellow line denote negative expression).
Discussion
At present, gastric cancer is a common disease world-wide, with a poor prognosis and low survival rates. To prevent this disease, we investigated the difference in protein expression between a major precancerous lesion namely varioliform gastritis (VG) [6] and the morphologically normal mucosa tissues near the lesions using the proteomic analysis. Our result shows that the expressions of S100A6 and S100A10 were upregulated in the precancerous lesion. They could be novel proteins in those differentially expressed proteins and involve cell cycle, cell death or proliferation-modifying processes in this precancerous change. But their relationships with clinicopathologic characteristics are poorly understood.
It was reported previously that S100A6 expression has been directly correlated with the neoplastic phenotype, being more expressed in cancer than in normal tissues in pancreas [40], thyroid [41] and colorectal [42] cancers. But, with regard to the expression of S100A6 in gastric cancer, just a few investigations were performed, furthermore the clinicopathologic significance of S100A6 was not provided. The results of the current research suggested that S100A6 gene were generally expressed in gastric adenocarcinoma and upregulated expression of S100A6 could have close relationship with more poorly differentiated adenocarcinoma, more lymph node metastasis and deeper invasion of tumor. Komatsu et al. reported that it was more expressed in liver metastasis then in primary tumors in colorectal cancer [42].This result could showed that an increased S100A6 level in tumor cells could couple with poor survival. The reason could be that the molecular and biologic factors that control the balance between cell proliferation and apoptosis in development and progression of gastric cancers could impacted by the changes of S100A6 gene expression. In some researches, the S100A6 protein has been implicated in the regulation of cell growth and proliferation because its mRNA is preferentially expressed in the G1 phase of the cell cycle [43]. The effects of S100A6 on cell cycling are probably mediated via interactions with various ligands including Calcyclin- binding protein (CacyBP/SIP),Cyclin-dependent kinase 5(CDK5), glyceraldehyde-3-phosphate dehydrogenase, and a 30 kDa protein present in Ehrlich ascites tumour cells [44-47] et al. The results of many previous researches suggests that S100A6 have an effective influence on growth and proliferation of gastric cancer cell, but the exact molecular mechanism of the influence is still unknown, so we will perform further research to investigate the functional role of the gene in vitro.
It was showed that the upregulated S100A10 could be significantly correlated with the proportion of lymph node metastasis and the depth of the tumors in gastric cancer. This result could suggest S100A10 plays the role as a tumorenhancing gene in gastric cancer in some distance. There is few previous report on the role of S100A10 in gastric cancer. Zhang et al. have confirmed that the growth and invasion of breast cancer cell could be restrained when the expression of S100A10 gene was downregulated using RNAi method [33]. It is well known that S100A10 protein can form a heterotetrameric complex with annexin 2 and this complex is thought to serve much function in cell, such as bridging or scaffolding function in the membrane underlying cytoskeleton. Zobiack et al. and Kwon et al. considered that both S100A10 and S100A10/annexin 2 complex play important roles in plasminogen regulation which could influent many functions correlated with cancer cell invasiveness and metastasis [48,49]. But the mechanism by which S100A10 and S100A10/annexin 2 complexes regulate plasminogen regulation and impact cancer cell invasiveness and metastasis is still not very clear, so the further molecular study should be conducted.
In conclusion, immunohistochemical expression of S100A6 and S100A10 could be correlated with poor differentiation of the tumors and poor prognosis of patients with gastric cancer. The further research should highlight the important mechanism that these gene play in cancer cell invasiveness and metastasis.
Acknowledgements
The authors wish to thank Drs Xuejuan Bai for handling patient contacts. We wish to thank the Forth Military Medical University of PLA for providing means for the current investigation.
References
- Parkin DM, Bray F, Ferlay J and Pisani P. Global cancer statistics,2002. CA Cancer J Clin 2005; 55: 74–108,.
- Crew KD, Neugut AI: Epidemiology of gastric cancer. World J Gastroenterol 2006; 12: 354-62.
- Correa P: Human gastric carcinogenesis: a multistep and multifactorial process -First American Cancer society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992; 52:6735-6740.
- Gonzalez CA, Sala N and Capella G. Genetic susceptibility and gastric cancer risk. Int J Cancer 2002; 100: 249-260.
- Xue FB, Xu YY, Wan Y. Association of H. pylori infection with gastric carcinoma: a Meta-analysis. World J Gastroenterol 2001; 7: 801-804.
- Zhang L,Hou YH,Wu K.Proteomic analysis reveals molecular biological details in varioliform gastritis without Helicobacter pylori infection. WJG 2010; 16:3664-3673.
- Schafer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology, Trends Biochem. Sci 1996; 21: 134-140.
- Lewit-Bentley A, Rety S. EF-hand calcium-binding proteins, Curr Opin Struct Biol 2000; 10: 637-643.
- Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 2001; 33: 637-668.
- Heizmann CW, Fritz G, Schafer BW. S100 proteins: structure, functions and pathology, Front. Biosci 2002; 7: d1356–d1368.
- Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun 2004; 322:1111–1122.
- Moore BW. A soluble protein characteristic of the nervous system. Biochem. Biophys Res Commun 1965; 19: 739-744.
- Mani RS, McCubbin WD, Kay CM. Calcium-dependent regulation of caldesmon by an 11-kDa smooth muscle calcium-binding protein, caltropin. Biochemistry 1992; 31: 11896-11901.
- Sorci G, Agneletti AL, Bianchi R, Donato R. Association of S100B with intermediate filaments and microtubules in glial cells. Biochim Biophys Acta 1998; 1448: 277-289.
- Garbuglia M, Verzini M, Sorci G, Bianchi R, Giambanco I, Agneletti AL. The calcium-modulated proteins, S100A1 and S100B, as potential regulators of the dynamics of type III intermediate filaments. Braz J Med Biol Res 1999; 32: 1177-1185.
- Golitsina NL, Kordowska J, Wang CL, Lehrer SS. Ca2C dependent binding of calcyclin to muscle tropomyosin, Biochem Biophys Res Commun 1996; 220: 360-365.
- Cormier K,Harquail J, Ouellette RJ. Intracellular expression of inflammatory proteins S100A8 and S100A9 leads to epithelial-mesenchymal transition and attenuated aggressivity of breast cancercells.Anticancer Agents Med Chem ,2014; 14:35-45.
- Chen H,Xu C, Jin Q. S100 protein family in human cancer.Am J Cancer Res 2014; 4:89-115.
- Chen N,Sato D,Saiki Y. S100A4 is frequently overexpressed in lung cancer cells and promotes cell growth and cell motility. Biochem Biophys Res Commun 2014; 447: 459-464.
- Zhu L,Okano S,Takahara M,et al:Expression of S100 protein family members in normal skin and sweat gland tumors. J Dermatol Sci 2013; 70: 211-219.
- Hirschhorn RR, Aller P, Yuan ZA, Gibson CW. Cell-cycle-specific cDNAs from mammalian cells temperature sensitive for growth. Proc Natl Acad Sci USA 1984; 81: 6004-6008.
- Kilanczyk E,Graczyk A,Ostrowska H,et al:S100A6 is transcriptionally regulated by beta-catenin and interacts with a novel target, lamin A/C, in colorectal cancer cells. Cell Calcium 2012; 51:470-477.
- Hua Z,Chen J, Sun B. Specific expression of osteopontin and S100A6 in hepatocellular carcinoma. Surgery 2011; 149: 783-91.
- Ohuchida K,Mizumoto K,Ishikawa N,et al:The role of S100A6 in pancreatic cancer development and its clinical implication as a diagnostic marker and therapeutic target. Clin Cancer Res 2005; 11: 7785-77893.
- Sato Y,Harada K,Sasaki M.Clinicopathological significance of S100 protein expression in cholangiocarcinoma.J Gastroenterol Hepatol 2013; 28: 1422-1429.
- Melle C,Ernst G,Schimmel B. Colon-derived liver metastasis, colorectal carcinoma, and hepatocellular carcinoma can be discriminated by the Ca(2+)-binding proteins S100A6 and S100A11.PLoS One 2008; 3: e3767.
- Zhu L,Okano S, Takahara M. Expression of S100 protein family members in normal skin and sweat gland tumors.J Dermatol Sci 2013; 70: 211-219.
- Sofiadis A,Dinets A,Orre LM. Proteomic study of thyroid tumors reveals frequent up-regulation of the Ca2+-binding protein S100A6 in papillary thyroid carcinoma.Thyroid 2010; 20: 1067-1076.
- Yang YQ, Zhang LJ, Dong H. Upregulated expression of S100A6 in human gastric cancer. J Dig Dis 2007; 8: 186-93.
- Ning X, Sun S, Hong L. Calcyclin-binding protein inhibits proliferation, tumorigenicity, and invasion of gastric cancer. Mol Cancer Res 2007; 5: 1254-1262.
- Rand V,Prebble E,Ridley L,Howard M. Investigation of chromosome 1q reveals differential expression of members of the S100 family in clinical subgroups of intracranial paediatric ependymoma. Br J Cancer 2008; 99: 1136-1143.
- Zhang J, Guo B, Zhang Y, Cao J. Silencing of the annexin II gene down-regulates the levels of S100A10, c-Myc, and plasmin and inhibits breast cancer cell proliferation and invasion. Saudi Med J 2010; 31: 374-381.
- Teratani T,Watanabe T,Kuwahara F,Kumagai H,Kobayashi S. Induced transcriptional expression of calcium-binding protein S100A1 and S100A10 genes in human renal cell carcinoma. Cancer Lett 2002; 175: 71-77.
- Rust R,Visser L, van der Leij J. High expression of calcium-binding proteins, S100A10, S100A11 and CALM2 in anaplastic large cell lymphoma.Br J Haematol 2005; 131: 596-608.
- El-Rifai W,Moskaluk CA,Abdrabbo MK, Harper J. Gastric cancers overexpress S100A calcium-binding proteins. Cancer Res 2002; 62: 6823-6826.
- Tsai CJ,Herrera-Goepfert R,Tibshirani RJ,Yang S. Changes of gene expression in gastric preneoplasia following Helicobacter pylori eradication therapy. Cancer Epidemiol Biomarkers Prev 2006; 15: 272-280.
- Sobin LH, Wittekind C. TNM classification of malignant tumours. In:International Union Against Cancer. Wiley-Liss 1997; 54-58.
- Kase S, Osaki M, Honjo S, et al:Expression of cyclo-oxygenase-2 is correlated with high intratumoral microvessel density and low apoptotic index in human esophageal squamous cell carcinomas. Virchows Arch 2003; 442: 129-135.
- Nozoe T, Ezaki T, Kabashima A, Baba H. Significance of intratumoral microvessel density and low apoptotic index in human esophageal squamous cell carcinomas. Virchows Arch 2003; 442: 129-135.
- Vimalachandran D, Greenhalf W, Thompson C, Luttges J, Prime W, Campbell F. High nuclear S100A6 (calcyclin) is significantly associated with poor survival in pancreatic cancer patients. Cancer Res 2005; 65: 3218-3225.
- Brown LM, Helmke SM, Hunsucker SW, Netea-Maier RT, Chiang SA, Heinz DE. Quantitative and qualitative differences in protein expression between papillary thyroid carcinoma and normal thyroid tissue. Mol Carcinog 2006; 45: 613-626.
- Komatsu K, Kobune-Fujiwara Y, Andoh A, Ishiguro S, Hunai H, Suzuki N. Increased expression of S100A6 at the invading fronts of the primary lesion and liver metastasis in patients with colorectal adenocarcinoma. Br J Cancer 83:769–74,2000.
- Calabretta B, Kaczmarek L, Mars W. Cell-cycle-specific genes differentially expressed in human leukemias. Proc Natl Acad Sci USA 1985; 82: 4463-4467.
- Li J,Wang XH, Li ZY. Regulation mechanism study of S100A6 on invasion and metastasis in gastric cancer.Zhonghua Wei Chang Wai Ke Za Zhi 2013; 16: 1096-1101.
- Ning X,Sun S, Zhang K. S100A6 protein negatively regulates CacyBP/SIP-mediated inhibition of gastric cancer cell proliferation and tumorigenesis.PLoS One 7: e30185. 2012.
- Nowotny M,Spiechowicz M, Jastrzebska B. Calcium-regulated interaction of Sgt1 with S100A6 (calcyclin) and other S100 proteins. J Biol Chem 2003; 278: 26923-26928.
- Zeng FY, Gerke V, Gabius HJ:Identification of annexin II, annexin IV and glyceraldehydes3-phosphate dehydrogenase as calcyclin-binding proteins in bovine heart. Inter J Biochem 1998; 25: 1019-1025.
- Zobiack N, Gerke V, Rescher U: Complex formation and submembranous localization of annexin 2 and S100A10 in live HepG2 cells. FEBS Lett 2001; 500: 137-140.
- Kwon M,MacLeod TJ, Zhang Y, Waisman DM. S100A10, annexin A2, and annexin a2 heterotetramer as candidate plasminogen receptors. Front Biosci 2005; 10: 300-325.