ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 6
Synthesis, antimicrobial and anticancer activities of some naphthylthiazolylamine derivatives
1Department of Chemistry, Eskisehir Osmangazi University, Eskisehir, Turkey
2Department of Biology, Anadolu University, Eskisehir, Turkey
3Department of Pharmaceutical Chemistry, School of Pharmacy, Medipol University, Istanbul, Turkey
Accepted date: November 9, 2016
New 4-naphthyl-2-aminothiazole derivatives were synthesized. The newly synthesized compounds were screened for their in vitro antimicrobial and anticancer activities. The results indicated that compound 5b namely 2-(4-methylpiperidine-1-yl)-4-(naphthalene-2-yl) thiazole exhibited highest MIC value (62.5 μg/ml) against P. aeruginosa in the tested microorganisms. This compound showed equipotent antifungal effect on C. albicans and C. glabrata as compared with ketoconazole. Compounds 4c, 4d, 5a and 5f showed remarkably antifungal activity against C. albicans. In addition, anticancer activity and cytotoxicity studies were also carried out in Hep-G2 and A549 cell lines to examine the ability of inhibiting the cell growth for 4a-4f and 5a-5f compounds. The cell viability and anticancer activity were determined by MTT assay. 4a-4f compounds has led to an increase in cell proliferation in both cell lines (Hep-G2 and A549 cells), unlike 5a-5f series showed a weak anticancer activity.
Keywords
Thiazole, Naphthalene, Antimicrobial activity, Cytotoxicity, MTT assay.
Introduction
Thiazole derivatives are important heterocyclic compounds because of their biological activities, such as antibacterial [1-5], antifungal [3-9], antitumor [3,10-16], anticonvulsant [17], antitubercular [18,19]. Also aminothiazoles as well as 2- amino-4-arylthiazole derivatives are shown antimicrobial [20-22] activities and amino thiazoles are known to be ligands of oestrogen receptor [23] as well as a novel class of adenosine receptors antagonists [24]. In addition, cyclic seconder amine derivatives (thiomorpholine, morpholine etc.) containing an azole nucleus or aryl group are exhibited significant antimicrobial [25-33], anti-inflammatory [34], cytotoxic [35], antidepressant [36], antileukemic [37], antibiotic [38], antiurease [31] activities.
Furthermore, naphthalene derivatives are found to be associated with various biological activities such as cytotoxic, antimicrobial [39-43], antioxidant [41], antiinflammatory [44].
Based on the above reports we were synthesized a series of new naphthyl thiazolylamine derivatives based on Scheme 1. We considered the synthesis of new thiazole derivatives from dialkylaminothiocarbamide and 2-bromo-(naphthalene-1/2-yl) ethanone and tested them for their in vitro antimicrobial and anticancer activity. The antimicrobial properties of these naphthylthiazolylamine compounds were evaluated against various selected bacterial and fungal strains using the Minimum Inhibitory Concentration (MIC) method. In addition, cytotoxicity studies were also carried out in Hep-G2 and A549 cell lines to examine the ability of these compounds to inhibit the cell growth.
Materials and Methods
Chemistry
All chemicals were used without further purification. All melting points were measured by using an Electrothermal 9300 digital melting points apparatus. Spectroscopic data were recorded on the following instruments: a Perkin Helmer FTIR 100 spectrophotometer; an 1H NMR (nuclear magnetic resonance) Bruker DPX 500 NMR spectrophotometer. Chemical shift values are given in δ-scales; a Mass Spectrometry (MS) Agilent 1100 MSD spectrometer (Agilent Technologies, Palo Alto, CA); an elemental analysis were performed in a Thermo Finnigan Flash EA 1112 elemental analyser. The completion of reactions was checked by Thin Layer Chromatography (TLC) on silica gel coated aluminium sheets (silica gel 60 F254) using a mixture of ethyl acetate and petroleum ether (1:1 v/v). N-benzoyl-dialkylaminothiocarbamide (1a-1f) and dialkylaminothiocarbamide (2a-2f) series were prepared according to the methods described in the literature [45].
General procedure for the synthesis of the compounds
N-benzoyl-dialkylamino-thiocarbamides (1a-1f): Benzoyl chloride (0.1 mol, 11.6 ml) were reacted with ammonium thiocyanate (0.105 mol, 8 g) in acetone and the mixture was heated under reflux for 15 minutes. Heating was stopped and the appropriate seconder amine derivatives (0.105 mol) were stirred for 30 minutes at room temperature. Then, this mixture was refluxed for 5 minutes until boiling temperature and checked by TLC. After completing the reaction, it was poured onto excess cracked ice with vigorous stirring. The precipitate formed was filtered and washed with water and dried.
Dialkylaminothiocarbamides (2a-2f): The appropriate Nbenzoyl- dialkylamino-thiocarbamide (1a-1f) was added to solution of 10% aq NaOH and this mixture was refluxed at 150°C for 10 minutes. The mixture was poured onto excess ice and to reaction media was added excess 37% HCl. After ammonia was added until pH 8. The mixture was kept in a cool place until pure product collapsed. The obtained precipitation was filtered and dried.
2-Bromo-1-(naphthalene-1/2-yl) ethanone (3a and 3b): 1- (naphthalene-1/2-yl) ethanone (0.03 mol) was taken in 25 ml of acetic acid in a 100 ml two-necked round bottomed flask. A solution of bromine (0.01 mol) in acetic acid was prepared. The bromine solution was dropped into the flask for two hours and then this mixture was stirred for six hours. After TLC, the mixture was kept in a cool place. 2-bromo-1-(naphthalene-1- yl) ethanone (3a) was obtained as a liquid. 2-bromo-1- (naphthalene-2-yl) ethanone (3b) was obtained as a solid, the resulting solid mass was filtered and dried.
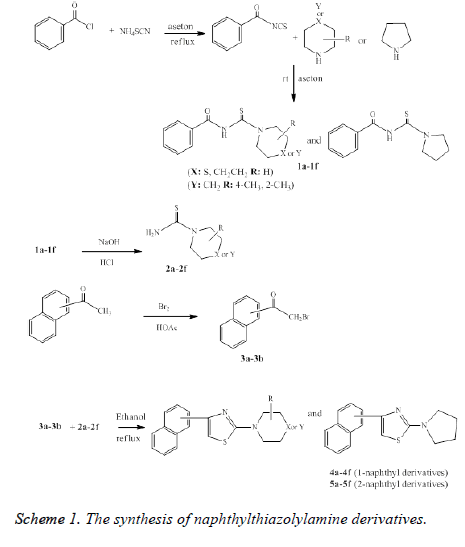
2-(Dialkylamino)-4-(naphthalene-1/2-yl) thiazoles (4a-4f and 5a-5f): A mixture of 2-bromo-1-(naphthalene-1/2-yl) ethanone (3a and 3b) (0.002 mol), appropriate dialkylaminothiocarbamide (2a-2f) (0.002 mol) in ethanol was refluxed for ten hours and checked by TLC. After completing the reaction, the cooled mixture to room temperature was filtered and dried. The physical properties and spectral data of the final compounds are given below.
2-(Piperidine-1-yl)-4-(naphthalene-1-yl) thiazole (4a): Yield 60%; mp. 153-154°C; FT-IR nmax (cm-1): 3049 (aromatic CH), 2917-2848 (aliphatic CH), 1613 (C=N), 1592, 1515, 1476 (C=C condense system), 1150-1100 (aliphatic amin), 630 (CS); 1H NMR (500 MHz, DMSO-d6) δ: 1.65 (6H, s, piperidine- H), 3.7 (4H, s, piperidine-H), 6.8 (1H, t, J: 7.71 Hz, naphthalene C3-H), 7.0 and 7.14 (1H, two t, J: 7.45 Hz, J: 7.63 Hz, naphthalene C6-H), 7.06 and 7.31 (2H, two d, J: 6.99 Hz, J: 7.08 Hz, naphthalene C2-H, naphthalene C7-H), 7.49-7.55 (2H, m, C5-H, thiazole-H), 7.57 and 7.85 (1H, two d, J: 6.8 Hz, J: 7.75 Hz, naphthalene C4-H), 7.77 and 7.97 (1H, two d, J: 8.19 Hz, J: 8.23 Hz, naphthalene C8-H), ppm; MS (ESI) (m/z): (M +H)+ 295; Analysed and Calculated for C18H18N2S (294.12): C, 73.43; H, 6.16; N, 9.51. Found: C, 73.88; H, 5.85; N, 9.46%.
2-(4-Methylpiperidine-1-yl)-4-(naphthalene-1-yl) thiazole (4b): Yield 35%; mp. 139-140°C; FT-IR nmax (cm-1): 3050 (aromatic CH), 2955-2861 (aliphatic CH), 1606 (C=N), 1505, 1472, 1456 (C=C condense system), 1150-1100 (aliphatic amin), 631 (C-S); 1H NMR (500 MHz, DMSO-d6) δ: 0.95 (3H, d, J: 6.44 Hz, CH3), 1.2-1.8 (5H, m, piperidine-H), 3.2 (2H, t, J: 11.59 Hz, piperidine-H), 4.1 (2H, s, piperidine-H), 6.8 (1H, t, J: 7.68 Hz, naphthalene C3-H), 7.0 and 7.14 (1H, two t, J:7.44 Hz, J: 7.63Hz, naphthalene C6-H), 7.06 and 7.31 (2H, two d, J: 6.85 Hz, J: 7.15Hz, naphthalene C2-H, naphthalene C7-H), 7.49-7.55 (2H, m, naphthalene C5-H, thiazole-H), 7.57 and 7.85 (1H, two d, J: 6.78 Hz, J: 7.82 Hz, naphthalene C4-H), 7.77 and 7.97 (1H, two d, J: 8.21 Hz, J: 8.25 Hz, naphthalene C8-H), ppm; MS (ESI) (m/z): (M+H)+ 309; Analysed and Calculated for C19H20N2S (308.13): C, 73.99; H, 6.54; N, 9.08. Found: C, 74.06; H, 5.98; N, 8.85%.
2-(2-Methylpiperidine-1-yl)-4-(naphthalene-1-yl) thiazole (4c): Yield 30%; mp. 148-149°C FT-IR nmax (cm-1): 3058, 3023 (aromatic CH), 2973-2864 (aliphatic CH), 1586 (C=N), 1508, 1471, 1440 (C=C condense system), 1150-1100 (aliphatic amin), 626 (C-S); 1H NMR (500 MHz, DMSO-d6) δ: 1.27 (3H, d, J: 6.8 Hz, CH3), 1.5-1.8 (6H, m, piperidine-H), 4.00 and 4.41 (1H, two s, piperidine-H), 3.2 (2H, t, J: 12.42 Hz, piperidine-H), 6.8 (1H, t, J: 7.62 Hz, naphthalene C3-H), 7.0 and 7.14 (1H, two t, J: 7.40 Hz, J: 7.63Hz, naphthalene C6-H), 7.06 and 7.31 (2H, two d, J: 6.96 Hz, J: 7.61 Hz, naphthalene C2-H, naphthalene C7-H), 7.49-7.55 (2H, m, naphthalene C5-H, thiazole-H), 7.57 vs. 7.85 (1H, two d, J: 6.83 Hz, J: 7.97 Hz, naphthalene C4-H), 7.77 and 7.97 (1H, two d, J: 8.17 Hz, J: 8.25 Hz, naphthalene C8-H), ppm; MS (ESI) (m/z): (M+H)+ 309; Analysed and Calculated for C19H20N2S (308.13): C, 73.99; H, 6.54; N, 9.08. Found: C, 74.15; H, 5.96; N, 8.86%.
2-(Pyrrolidin-1-il)-4-(naphthalene-1-yl) thiazole (4d): Yield 45%; mp. 142-143°C FT-IR nmax (cm-1): 3173, 3049 (aromatic CH), 2985-2820 (aliphatic CH), 1654 (C=N), 1589-1468 (C=C condense system), 1150-1100 (aliphatic amin), 624 (C-S); 1H NMR (500 MHz, DMSO-d6) δ: 2.1 (4H, s, pyrrolidin-H), 3.6 (4H, s, pyrrolidin-H), 6.8 (1H, t, J: 7.71 Hz, naphthalene C3-H), 6.9 and 7.15 (1H, two t, J: 7.45 Hz, J: 8.03 Hz, naphthalene C6- H), 7.04 and 7.29 (2H, two d, J: 6.95 Hz, J: 7.04Hz, naphthalene C2-H, naphthalene C7-H), 7.48-7.54 (2H, m, naphthalene C5-H, thiazole-H), 7.56 and 7.85 (1H, two d, J: 6.78 Hz, J: 7.63 Hz, naphthalene C4-H), 7.76 and 7,95 (1H, two d, J: 8.22 Hz, naphthalene C8-H), ppm; MS (ESI) (m/z): (M +H)+ 281; Analysed and Calculated for C17H16N2S (280.10): C, 72.82; H, 5.75; N, 9.99. Found: C, 73.06; H, 5.37; N, 9.61%.
2-(Hexamethyleneamin-1-yl)-4-(naphthalene-1-yl) thiazole (4e)
Yield 54%; mp. 119-120°C FT-IR nmax (cm-1): 3072 (aromatic CH), 2972-2885 (aliphatic CH), 1685 (C=N), 1581-1460 (C=C condense system), 1158-1131 (aliphatic amin), 621 (C-S); 1H NMR (500 MHz, DMSO-d6) δ: 1.6 (4H, s, hexamethyleneamin-H), 1.8 (4H, s, hexamethyleneamin-H), 2.2 (4H, s, hexamethyleneamin-H), 6.8 (1H, t, J: 7.7 Hz, naphthalene C3-H), 7.0 and 7.14 (1H, two t, J:7.46 Hz, J: 7.63 Hz, naphthalene C6-H), 7.06 and 7.3 (2H, two d, J: 6.97 Hz, J: 7.12 Hz, naphthalene C2-H, naphthalene C7-H), 7.49-7.55 (2H, m, naphthalene C5-H, thiazole-H), 7.57 and 7.85 (1H, two d, J: 5.85 Hz, J: 7.67 Hz, naphthalene C4-H), 7.77 and 7.97 (1H, two d, J: 8.21 Hz, J: 8.23 Hz, naphthalene C8-H), ppm; MS (ESI) (m/z): (M+H)+ 309; Analysed and Calculated for C19H20N2S (308.13): C, 73.99; H, 6.54; N, 9.08. Found: C, 74.34; H, 6.83; N, 8.98%.
2-(Thiomorpholine-4-yl)-4-(naphthalene-1-yl) thiazole (4f): Yield 40%; mp. 165-166°C FT-IR nmax (cm-1): 3043 (aromatic CH), 2965-2803 (aliphatic CH), 1622 (C=N), 1600-1477 (C=C condense system), 1144-1126 (aliphatic amine), 623 (C-S); 1H NMR (500 MHz, DMSO-d6) δ: 2.1 and 2.8 (4H, s, thiomorpholine-H), 3.9 (4H, s, thiomorpholine-H), 6.8 (1H, t, J: 7.62 Hz, naphthalene C3-H), 7.02 and 7.16 (1H, two t, J: 7.42 Hz, J: 7.63Hz, naphthalene C6-H), 7.08 and 7.33 (2H, two d, J: 6.94 Hz, J: 7.59 Hz, naphthalene C2-H, naphthalene C7-H), 7.5-7.54 (2H, m, naphthalene C5-H, thiazole-H), 7.57 and 7.86 (1H, two d, J: 6.88 Hz, J: 95 Hz, naphthalene C4-H), 7.78 and 7.98 (1H, two d, J: 8.18 Hz, J: 8.23 Hz, naphthalene C8-H), ppm; MS (ESI) (m/z): (M+H)+ 313; Analysed and Calculated for C17H16N2S2 (312.08): C, 65.35; H, 5.16; N, 8.97. Found: C, 66.9; H, 4.99; N, 8.7%.
2-(Piperidine-1-yl)-4-(naphthalene-2-yl) thiazole (5a): Yield 40%; mp. 149-150°C FT-IR nmax (cm-1): 3051 (aromatic CH), 2955-2831(aliphatic CH), 1628 (C=N), 1583-1443 (C=C condense system), 1129 (aliphatic amine), 626 (C-S); 1H NMR (500 MHz, DMSO-d6) δ: 1.7 (6H, s, piperidine-H), 3.7 (4H, s, piperidine-H), 6.97 (2H, t, J: 5.68 Hz, naphthalene C6,7-H), 7.29 -7.56 (3H, m, naphthalene C4,5-H, thiazole-H), 7.71 -7.86 (2H, m, naphthalene C3,8-H), 7.98 (1H, s, naphthalene C1-H), ppm; MS (ESI) (m/z): (M+H)+ 295; Analysed and Calculated for C18H18N2S (294.12): C, 73.43; H, 6.16; N, 9.51. Found: C, 73.86; H, 5.91; N, 9.46%.
2-(4-methylpiperidine-1-yl)-4-(naphthalene-2-yl) thiazole (5b): Yield 25%; mp. 124-125°C FT-IR nmax (cm-1): 3058-3040 (aromatic CH), 2955-2870 (aliphatic CH), 1606 (C=N), 1594-1442 (C=C condense system), 1155-1104 (aliphatic amine), 626 (C-S); 1H NMR (500 MHz, DMSO-d6) δ: 0.95 (3H, d, J: 6.48 Hz, CH3), 1.2-1.3 (2H, m, piperidine-H), 1.75 (2H, d, J: 12.75 Hz, piperidine-H), 3.2 (3H, t, J: 9.36 Hz, piperidine-H), 4.07 (2H, d, J: 11.07 Hz, piperidine-H), 6.97 (2H, t, J: 6.11 Hz, naphthalene C6,7-H), 7.29-7.56 (3H, m, naphthalene C4,5-H, thiazole-H), 7.71-7.86 (2H, m, naphthalene C3,8-H), 7.98 (1H, s, naphthalene C1-H), ppm; MS (ESI) (m/z): (M+H)+ 309; Analysed and calculated for C19H20N2S (308.13): C, 73.99; H, 6.54; N, 9.08. Found: C, 74.02; H, 6.01; N, 8.92%.
2-(2-Methylpiperidine-1-yl)-4-(naphthalene-2-yl) thiazole (5c): Yield 35% mp. 102-103°C FT-IR nmax (cm-1): 3056 (aromatic CH), 2940-2863 (aliphatic CH), 1652 (C=N), 1595-1436 (C=C condense system), 1164-1128 (aliphatic amine), 627 (C-S); 1H NMR (500 MHz, DMSO-d6) δ: 1.3 (3H, d, J: 6.81 Hz, CH3), 1.48-1.7 (6H, m, piperidine-H), 3.25 (1H, t, J: 11.15 Hz, piperidine-H), 3.9 (1H, d, J: 11.32 Hz, piperidine- H), 4.4 (1H, s, piperidine-H), 6.97 (2H, t, J: 5.65 Hz, naphthalene C6,7-H), 7.29-7.56 (3H, m, naphthalene C4,5-H, thiazole-H), 7.71-7.86 (2H, m, naphthalene C3,8-H), 7.98 (1H, s, naphthalene C1-H), ppm; MS (ESI) (m/z): (M+H)+ 309; Analysed and Calculated for C19H20N2S (308.13): C, 73.99; H, 6.54; N, 9.08. Found: C, 74.10; H, 5.99; N, 8.88%.
2-(Pyrrolidin-1-yl)-4-(naphthalene-2-yl) thiazole (5d): Yield 40% mp. 191-192°C; FT-IR nmax (cm-1): 3058 (aromatic CH), 2953-2851 (aliphatic CH), 1628 (C=N), 1583-1443 (C=C condense system), 1128 (aliphatic amine), 627 (C-S); 1H NMR (500 MHz, DMSO-d6) δ: 2.1 (4H, s, pyrrolidin-H), 3.55 (4H, s, pyrrolidin-H), 6.97 (2H, t, J: 5.12 Hz, naphthalene C6,7-H), 7.29-7.55 (3H, m, naphthalene C4,5-H, thiazole-H), 7.71-7.86 (2H, m, naphthalene C3,8-H), 7.98 (1H, s, naphthalene C1-H), ppm; MS (ESI) (m/z): (M+H)+ 281; Analysed and Calculated for C17H16N2S (280.10): C, 72.82; H, 5.75; N, 9.99. Found: C, 73.10; H, 5.39; N, 9.64%.
2-(Hexamethyleneamin-1-yl)-4-(naphthalene-2-yl) thiazole (5e): Yield 35%; mp. 132-133°C; FT-IR nmax (cm-1): 3062 (aromatic CH), 2939-2852 (aliphatic CH), 1606 (C=N), 1581-1442 (C=C condense system), 1148-1127 (aliphatic amine), 626 (C-S); 1H NMR (500 MHz, DMSO-d6) δ: 1.6 (4H, s, hexamethyleneamin-H), 1.8 (4H, s, hexamethyleneamin-H), 3, 7 (4H, s, hexamethyleneamin-H), 6.75 (2H, t, J: 6.19 Hz, naphthalene C6,7-H), 7.29-7.55 (3H, m, naphthalene C4,5-H, thiazole-H), 7.71-7.85 (2H, m, naphthalene C3,8-H), 7.98 (1H, s, naphthalene C1-H), ppm; MS (ESI) (m/z): (M+H)+ 309; Analysed and Calculated for C19H20N2S (308.13): C, 73.99; H, 6.54; N, 9.08. Found: C, 74.32; H, 6.78; N, 8.96%.
2-(Thiomorpholine-4-yl)-4-(naphthalene-2-yl) thiazole (5f): Yield 40%; mp. 194-195°C; FT-IR nmax (cm-1): 3049 (aromatic CH), 2960-2852 (aliphatic CH), 1628 (C=N), 1583-1474 (C=C condense system), 1174-1127 (aliphatic amine), 626 (C-S); 1H NMR (500 MHz, DMSO-d6) δ: 2.8 (4H, m, thiomorpholine- H), 3.9 (4H, s, thiomorpholine-H), 6.97 (2H, t, J: 5.29 Hz, naphthalene C6,7-H), 7.29 -7.55 (3H, m, naphthalene C4,5-H, thiazole-H), 7.73-7.86 (2H, m, naphthalene C3,8-H), 8.1 (1H, s, naphthalene C1-H), ppm; MS (ESI) (m/z): (M+H)+ 313; Analysed and Calculated for C17H16N2S2 (312.08): C, 65.35; H, 5.16; N, 8.97. Found: C, 66.5; H, 5.02; N, 8.74%.
Antimicrobial activity
The antimicrobial activity of the compounds was evaluated by micro-broth dilution method [46]. All compounds were tested for their in vitro antimicrobial activity against Staphylococcus aureus (ATCC-33862), Bacillus cereus (NRRL B-3711), Listeria monocytogenes (ATCC-7644), Escherichia coli (ATCC-25922), Pseudomonas aeruginosa (ATCC-27853), Salmonella typhimurium (NRRL B-4420), Candida albicans (Clinical isolate, Osmangazi University, Faculty of Medicine, Eskisehir, Turkey) and Candida glabrata (Clinical isolate, Osmangazi University, Faculty of Medicine, Eskisehir, Turkey).
Stock solutions of tested compounds were prepared in Dimethyl Sulfoxide (DMSO). Two-fold serial dilutions of the solutions using sterile distilled water were prepared from 2 mg/ml to 0.0039 mg/ml in micro-test tubes that were transferred to 96 well microtiter plates. After overnight incubation in double-strength Mueller-Hinton broth microbial suspensions were standardised to 108 CFU/ml using 0.5 McFarland solutions. One hundred microliter of each microorganism suspension was added the micro-plate wells containing of compound concentrations. The last two well on the micro-plates were used as controls. One was used as negative control containing uninoculated medium. The other well is containing sterile distilled water and inoculated medium as a positive growth control. The plates were incubated at 37°C for 18-24 h. After then, antimicrobial activity was detected by spraying of 0.5% TTC (Triphenyl Tetrazolium chloride, Merck) aqueous solution. Minimum Inhibitory Concentration (MIC) was determined as the lowest concentration of the compounds that inhibited visible growth. Streptomycin was used as standard antibacterial agent and ketoconazole as an antifungal agent. All compounds tested were screened triplicate against each microorganism.
In-vitro cell viability and anti-tumoral activity
Cell line, culture conditions and preparation of compounds: Human hepatocarcinoma cells (Hep-G2) and human lung adenocarcinoma (A549) cells were used to determine the cytotoxic properties. Cell lines Hep-G2 and A549 were used from the cell line collections of Anadolu University (Eskisehir, Turkey). Hep-G2 cell lines were cultivated in Dulbecco’s Modified Eagle’s Medium (DMEM), while A549 cells were grown RPMI-1640 mediums. All of the growth media were supplemented with 10% Foetal Bovine Serum (FBS) and antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin) at 37°C in a humidified atmosphere containing 5% CO2. Confluent adherent cultures were detached using trypsin-EDTA and were propagated in subcultures [47]. All compounds and Doxorubicin as a positive control were dissolved in DMSO (Dimethylsulfoxide) and final concentrations were diluted in culture medium, maximum DMSO concentration was adjusted to 0.1% per well.
Viability assay and antitumor activity
Viability of the test compounds-treated cells was evaluated by MTT (3-(4, 5-dimethylthiazol-2-il)-2, 5-diphenyltetrazolium bromide) assay as previously described by Mossman et al. [48]. The principle of the MTT method is based on the conversion of the yellow coloured tetrazolium salt (MTT dye) to purple formazan crystals by the enzymes in living cells and results are evaluated by spectrophometrically measurement of the intensity of the purple [49].
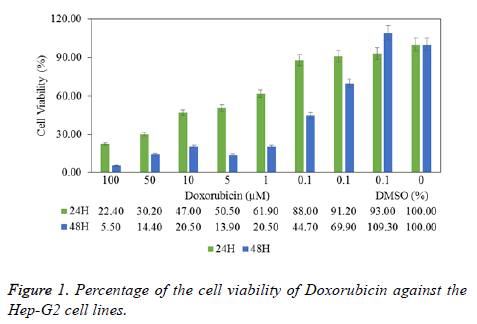
The cells were grown on to 96-well plates at the 1 × 104 for Hep-G2 and 4 × 103 for A549 cells per well and it was incubated for 24 h in culture conditions. Culture medium was replaced with fresh medium containing test compounds (4a-4f, 5a-5f) at different concentrations (12.5-200 μM). After 24 and 48 h incubations, cells were treated with fresh medium supplemented MTT (1 mg/ml) for 3 h at 37°C in incubator containing 5% CO2. Following the 4 h, medium was discarded and formazan crystals were dissolved in 200 μL DMSO per well for 20 min on a shaker at 1600 rpm and room temperature in dark. The viability of the cell was determined by measuring the Optical Density (OD) of each well as an absorbance at 570 nm using a micro-plate reader (ELX 808IU, Biotek Ins. Inc., Winooski, VT, USA). IC50 value was calculated [50]. Obtained absorbance value was evaluated by comparing the solvent control (0.1% DMSO treatment cells) and negative control (non-treatment cells). Doxorubicin was uses as reference standard at different concentrations (0.1-100 μM). Percent of cell viability compared to the negative control was shown graphically in Microsoft Office Professional Plus 2013 Excel program and it is given in Figures1-3.
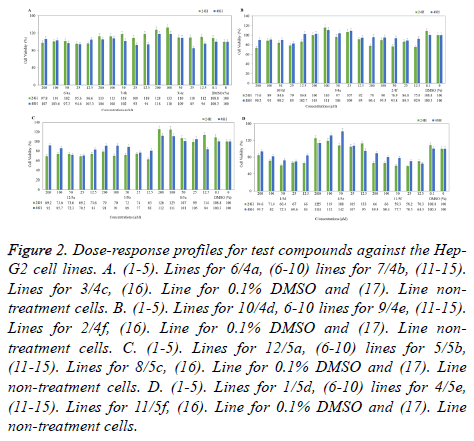
Figure 2. Dose-response profiles for test compounds against the Hep- G2 cell lines. A. (1-5). Lines for 6/4a, (6-10) lines for 7/4b, (11-15). Lines for 3/4c, (16). Line for 0.1% DMSO and (17). Line nontreatment cells. B. (1-5). Lines for 10/4d, 6-10 lines for 9/4e, (11-15). Lines for 2/4f, (16). Line for 0.1% DMSO and (17). Line nontreatment cells. C. (1-5). Lines for 12/5a, (6-10) lines for 5/5b, (11-15). Lines for 8/5c, (16). Line for 0.1% DMSO and (17). Line non-treatment cells. D. (1-5). Lines for 1/5d, (6-10) lines for 4/5e, (11-15). Lines for 11/5f, (16). Line for 0.1% DMSO and (17). Line non-treatment cells.
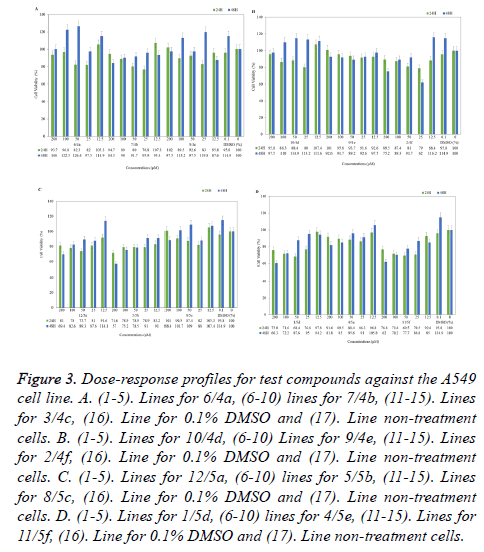
Figure 3. Dose-response profiles for test compounds against the A549 cell line. A. (1-5). Lines for 6/4a, (6-10) lines for 7/4b, (11-15). Lines for 3/4c, (16). Line for 0.1% DMSO and (17). Line non-treatment cells. B. (1-5). Lines for 10/4d, (6-10) Lines for 9/4e, (11-15). Lines for 2/4f, (16). Line for 0.1% DMSO and (17). Line non-treatment cells. C. (1-5). Lines for 12/5a, (6-10) lines for 5/5b, (11-15). Lines for 8/5c, (16). Line for 0.1% DMSO and (17). Line non-treatment cells. D. (1-5). Lines for 1/5d, (6-10) lines for 4/5e, (11-15). Lines for 11/5f, (16). Line for 0.1% DMSO and (17). Line non-treatment cells.
Results and Discussion
Chemistry
4-naphthyl-2-aminothiazole derivatives were synthesized as indicated in Scheme 1. The compounds N-benzoyldialkylamino- thiocarbamide (1a-1f), dialkylaminothiocarbamide (2a-2f) were synthesized, in accordance with the method described in literature [45]. The synthesis of Nbenzoyl- dialkylamino-thiocarbamide (1a-1f) was performed from the reaction of benzoyl chloride with ammonium thiocyanate in acetone. The obtained N-benzoyl-dialkylaminothiocarbamide was reacted with secondary amines (morpholine, thiomorpholine, piperidine, pyrrolidine, hexamethyleneamine) and then it was converted to the corresponding dialkylamino thiocarbamide (2a-2f) by hydrolysis.
2-bromo-(1- or 2-naphthyl) ethanone compounds (3a and 3b) were prepared by reacting 1- or 2-acetyl naphthalene and bromine in acetic acid.
4-naphthyl-2-aminothiazole derivatives (4a-4f and 5a-5f) were synthesized by reacting dialkyl thiocarbamide (2a-2f) and 2- bromo-(1- or 2-naphthyl) ethanone (3a and 3b) in ethanol.
The structures of the final compounds (4a-4f and 5a-5f) obtained were elucidated using spectral data. In the IR spectra of the compounds, characteristic absorbtion bands in the range of 1586-1685 cm-1, 1436-1600 cm-1, 1100-1174 cm-1 corresponding to-C=N-stretching of thiazole, -C=C- condense system stretching of naphthalene ring and –C-N-C- stretching of aliphatic aminee respectively. 1H NMR spectra of all the synthesized 4-naphthyl-2-aminothiazoles (4a-4f and 5a-5f), observed at δ 6.75-7.98 of naphthalene ring protons and δ 7.49-7.56 of thiazole ring proton in similar range. The secondary amine protons linked to thiazole were observed at δ 0.95-4.41.
Antimicrobial activity
All the newly synthesized compounds were evaluated for their in vitro antimicrobial activities against S. aureus, B. cereus, L. monocytogenes (as examples Gram positive bacteria), E. coli, P. aeruginosa, S. typhimurium (as examples Gram negative bacteria), C. albicans and C. glabrata (as examples yeasts) using streptomycin and ketoconazole as control drugs. The results are presented in Table 1.
| Microorganisms | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compounds | A | B | C | D | E | F | G | H |
| 4a | 500 | 500 | 500 | 500 | 500 | 500 | 1000 | 1000 |
| 4b | 500 | 500 | 500 | 500 | 500 | 500 | 1000 | 1000 |
| 4c | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 |
| 4d | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 |
| 4e | 500 | 500 | 500 | 500 | 500 | 500 | 1000 | 1000 |
| 4f | 500 | 500 | 500 | 500 | 500 | 500 | 1000 | 1000 |
| 5a | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 1000 |
| 5b | 125 | 125 | 250 | 250 | 62.5 | 250 | 250 | 250 |
| 5c | 500 | 500 | 500 | 500 | 500 | 500 | 1000 | 1000 |
| 5d | 500 | 500 | 500 | 500 | 500 | 500 | 1000 | 1000 |
| 5e | 500 | 500 | 500 | 500 | 500 | 500 | 1000 | 1000 |
| 5f | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 1000 |
| Streptomycin | 15.625 | 31.25 | 7.81 | 31.25 | 31.25 | 31.25 | — | — |
| Ketoconazole | — | — | — | — | — | — | 250 | 250 |
| A: Staphylococcus aureus (ATCC-33862); B: Bacillus cereus (NRRL B-3711); C: Listeria monocytogenes (ATCC-7644); D: Escherichia coli (ATCC-25922); E: Pseudomonas aeruginosa (ATCC-27853); F: Salmonella typhimurium (NRRL B-4420); G: Candida albicans (Clinical isolate); H: Candida glabrata (Clinical isolate). | ||||||||
Table 1. Antimicrobial activity of the compounds (4a-4f and 5a-5f) (μg/ml).
In general, all compounds demonstrated better antibacterial activity than antifungal activity. The results indicated that the compound 5b exhibited the highest MIC value (62.5 μg/ml) against P. aeruginosa in the tested microorganisms. Compound 5b showed equipotent antifungal effect on C. albicans and C. glabrata as compared with ketoconazole with a MIC value of 250 μg/ml. All compounds (4a-4f and 5a-5f) showed less antimicrobial activity than streptomycin.
All compounds except compound 5b are equally active (MIC=500 μg/ml) against all of the tested bacteria. Compounds 4c, 4d, 5a and 5f showed significant antifungal activity against C. albicans with a MIC value of 500 μg/ml. Among the compounds, 5b was the most susceptible compound against the all of the tested microorganisms.
In-vitro cell viability and anticancer activity
In vitro cell culture studies were performed in the Cell Culture Laboratory, Faculty of Sciences, Department of Biology in Anadolu University, Eskisehir, Turkey. Compounds 4a-4f and 5a-5f were analysed in terms of cytotoxicity and antitumor activity against human hepatocellular carcinoma (Hep-G2) and human lung adenocarcinoma (A549) cells. Doxorubicin was tested as a reference chemotherapeutic drugs and it showed IC50 values 5 μM for 24 h and 0.5 μM for 48 h in Hep-G2 cells (Figure 1).
When the cytotoxicity and anticancer properties of compounds were evaluated towards to Hep-G2 hepatocarcinoma cells, some compounds showed quite variable effects (Figure 2).
IC50 values could not found in any compound. However, compounds 5f and 5d were determined the most cytotoxic and anticancer potential compounds amongst others. After the 24 h incubation, when the treatment 200 μM compound 5f, cell viability was determined 66%. On the other hand, in 25 μMtreated cells was observed around 58% viability and after 24 and 48 hours, in 12.5 μM-treated cells showed 70% and 65% respectively. Similarly, compound 5d showed equipotent anticancer activity against hepatocarcinoma cells, 50 μM compound 5d treated cells exhibited 60% viability, in applied to 12.5 μM, viability was seen about 67% (Figure 2D). In addition, viability was about 70% in cells exposed to compound 4f and 5a (Figures 2B and 2C). Other compounds (4a-4d and 5c) were not effective on anticancer properties against hepatocarcinoma cell. However, compounds 4b, 4c and 5e slightly increased the cell proliferation at 24 h in Hep-G2 cells (Figures 2A-2C).
Considering the human lung adenocarcinoma cell lines (A549), only compound 5b with anticancer activity are noteworthy with 57% viability in 200 μM for 48 h, other concentrations of 5b were not cytotoxic (Figure 3C). Other compounds of the series 5a-5f; compounds 5a, 5d and 5f have led to 60, 62 and 69 % viability at 48 hour, respectively (Figure 3C and 3D). The compound 5c also did not cause an effect on cell proliferation. Further; almost all compounds of the series 4a-4f (excluding 4f) has no cytotoxic effects. In contrast, 4c and 4d compounds slightly increased the cell proliferation at 48 hours in lung cancer cells. The other hand, cells were exposed to 25 μM compound 4f showed 62% viability at 48 h (Figure 3A and 3B).
Conclusion
The synthesis of new 4-naphthyl-2-aminothiazole derivatives and evaluating their antimicrobial and anticancer activity has been investigated, in this study. The antimicrobial screening has revealed that compound 5b has remarkable antimicrobial activity, especially against P. aeruginosa. In addition, anticancer activities were evaluated against Hep-G2 and A549 tumor cell lines. When 4a-4f and 5a-5f anticancer profiles were compared, derivatives of series 5a-5f (excluding 5c) showed a weak anticancer activity. In contrast, the results indicate that; series 4a-4f has increased cell proliferation in both hepatocarcinoma and lung adenocarcinoma cells. When all data are compared with doxorubicin as standard chemotherapeutic drug (IC50 0.5 μM at 48 h) (Figure 1), it has been seen that compounds of 5a-5f have poor chemotherapeutic potential.
Conflict of Interests
The authors have declared no conflict of interests
Acknowledgements
This work was supported by the Commission of Scientific Research Projects of Eskisehir Osmangazi University (ESOGU/201219A108). The authors gratefully acknowledge the financial support by Eskisehir Osmangazi University.
References
- Kalhor M, Salehifar M, Nikokar I. Synthesis, characterization, and antibacterial activities of some novel N, N-disubstituted thiourea, 2-amino thiazole, and imidazole-2-thione derivatives. Med Chem Res 2014; 23: 2947-2954.
- Tsuji K, Ishikawa H. Synthesis and anti-pseudomonal activity of new 2-isocephems with a dihydroxypyridone moiety at C-7. Bioorg Med Chem Lett 1994; 4: 1601-1606.
- Rostom SAF, Faidallah HM, Radwan MF, Badr MH. Bifunctional ethyl 2-amino-4-methylthiazole-5-carboxylate derivatives: Synthesis and in vitro biological evaluation as antimicrobial and anticancer agents. Eur J Med Chem 2014; 76: 170-181.
- Karegoudar P, Karthikeyan MS, Prasad DJ, Mahalinga M, Holla BS. Synthesis of some novel 2, 4-disubstituted thiazoles as possible antimicrobial agents. Eur J Med Chem 2008; 43: 261-267.
- Desai NC, Bhatt N, Somani H, Trivedi A. Synthesis, antimicrobial and cytotoxic activities of some novel thiazole clubbed 1, 3, 4-oxadiazoles. Eur J Med Chem 2013; 67: 54-59.
- Wilson KJ, Illig CR, Subasinghe N, Hoffman JB, Rudolph MJ, Soll R, Molloy CJ, Bone R, Green D, Randall T, Zhang M, Lewandowski FA, Zhou Z, Sharp C, Maguire D, Grasberger B, DesJarlais RL, Spurlino J. Synthesis of thiophene-2-carboxamidines containing 2-aminothiazoles and their biological evaluation as urokinase inhibitors. Bioorg Med Chem Lett 2001; 11: 915-918.
- Omar K, Geronikaki A, Zoumpoulakis P, Camoutsis C, Sokovia M. Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg Med Chem 2010; 18: 426-432.
- Chimenti F, Bizzarri B, Bolasco A, Secci D, Chimenti P. Synthesis and biological evaluation of novel 2, 4-disubstituted-1, 3-thiazoles as anti-Candida spp. agents. Eur J Med Chem 2011; 46: 378-382.
- Darji ND, Pasha TY, Bhandri A, Molvi KI, Desai SA, Makwana MV. Synthesis of some novel 2, 4, 5-trisubstituted thiazoles as possible antifungal agents. J Pharm Res 2011; 4: 4465-4466.
- Kumar Y, Green R, Wise DS, Wotring LL, Townsend LB. Synthesis of 2, 4-disubstituted thiazoles and selenazoles aspotential antifilarial and antitumor agents. 2. 2-arylamido and 2-alkylamido derivatives of 2-amino-4-(isothiocyanatomethyl) thiazole and 2-amino-4-(isothiocyanatomethyl) selenazole. J Med Chem 1993; 36: 3849-3852.
- Gouda MA, Berghot MA, Baz EA, Hamama WS. Synthesis, antitumor and antioxidant evaluation of some new thiazole and thiophene derivatives incorporated coumarin moiety. Med Chem Res 2012; 21: 1062-1107.
- Dawood KM, Eldebss TM, El-Zahabi HS, Yousef MH, Metz P. Synthesis of some new pyrazole-based 1,3-thiazoles and 1,3,4-thiadiazoles as anticancer agents. Eur J Med Chem 2013; 70: 740-749.
- Srimanth K, Rao VR, Krishna DR. Synthesis and evaluation of anticancer activity of some imidazothiazolyl, imidazobenzothiazolyl and dihydroimidazothiazolyl coumarins. Arzneimittel Forsch 2002; 52: 388-392.
- Hay MP, Turcotte S, Flanagan JU, Bonnet M, Chan DA, Sutphin PD, Nguyen P, Giaccia AJ, Denny WA. 4-Pyridylanilinothiazoles that selectively target von Hippel-Lindau deficient renal cell carcinoma cells by inducing autophagic cell death. J Med Chem 2010; 53: 787-797.
- Al-Said MS, Bashandy MS, Al-Qasoumi SI, Ghorab MM. Anti-breast cancer activity of some novel 1, 2-dihydropyridine, thiophene and thiazole derivatives. Eur J Med Chem 2011; 46: 137-141.
- Romagnoli R, Baraldi PG, Salvador MK, Preti D, Tabrizi MA, Brancale A, Fu XH, Li J, Zhang SZ, Hamel E, Bortolozzi R, Porcu E, Basso G, Viola G. Discovery and optimization of a series of 2-aryl-4-amino-5-(3, 4, 5-trimethoxybenzoyl) thiazoles as novel anticancer agents. J Med Chem 2012; 55: 5433-5445.
- Ergenc N, Capan G. Synthesis and anticonvulsant activity of new 4-thiazolidone and 4-thiazoline derivatives. Farmaco 1994; 49: 449-451.
- Suresh Kumar GV, Rajendraprasad Y, Mallikarjuna BP, Chandrashekar SM, Kistayya C. Synthesis of some novel 2-substituted-5-(isopropylthiazole) clubbed 1, 2, 4-triazole and 1, 3, 4-oxadiazoles as potential antimicrobial and antitubercular agents. Eur J Med Chem 2010; 45: 2063-2074.
- Makam P, Kankanala R, Prakash A, Kannan T. 2-(2-Hydrazinyl) thiazole derivatives: Design, synthesis and in vitro antimycobacterial studies. Eur J Med Chem 2013; 69: 564-576.
- Morales-Bonilla P, Perez-Cardena A, Quintero-Marmol E, Arias-Tellez JL, Mena-Rejon GJ. Preparation, antimicrobial activity, and toxicity of 2-amino-4-arylthiazole derivatives. Heteroatom Chem 2006; 17: 254-260.
- Pattan SR, Shamrez M, Pattan JS, Purohit SS, Reddy VVK, Nataraj BR. Synthesis and microbiological evaluation of 2-acetanilido-4-arylthiazole derivatives. Ind J Chem 2006; 45: 1929-1932.
- Dighe SN, Chaskar PK, Jain KS, Phoujdar MS, Srinivasan KV. A remarkably high-speed solution-phase combinatorial synthesis of 2-substituted-amino-4-aryl thiazoles in polar solvents in the absence of a catalyst under ambient conditions and study of their antimicrobial activities. ISRN Org Chem 2011; 1-6.
- Fink BE, Mortensen DS, Stauffer SR, Aron ZD, Katzenellenbogen JA. Novel structural templates for estrogenreceptor ligands and prospects for combinatorial synthesis of estrogens. Chem Biol 1999; 6: 205-219.
- Van Muijlwijk-Koezen JE, Timmerman H, Vollinga RC, Frijtag von Drabbe Kunzel J, de Groote M, Visser S, IJzerman AP. Thiazole and thiadiazole analogues as a novel class of adenosine receptor antagonists. J Med Chem 2001; 44: 749-762.
- Desai NC, Rajpara KM, Joshi VV, Vaghani HV, Satodiya HM. Synthesis of promising antimicrobial agents: a novel series of N-(4-(2,6-dichloroquinolin-3-yl)-6-(aryl)pyrimidin-2-yl)-2-morpholinoacetamides. Med Chem Res 2013; 22: 1172-1183.
- Govindaraju R, Gopalakrishnan M, Thanusu J, Kanagarajan V. Synthesis, antibacterial, and antifungal activities of biolabile (E)-1-(4-morpholinophenyl)-3-aryl-prop-2-en-1-ones. Med Chem Res 2009; 18: 341-350.
- Singh U, Raju B, Lam S, Zhou J, Gadwood RC, Ford CW, Zurenko GE, Schaadt RD, Morin SE, Adams WJ, Friis JM, Courtney M, Palandra J, Hackbarth CJ, Lopez S, Wu C, Mortell KH, Trias J, Yuan Z, Patel DV, Gordeev MF. New antibacterial tetrahydro-4(2H)-thiopyran and thiomorpholine S-oxide and S, S-dioxide phenyloxazolidinones. Bioorg Med Chem Lett 2003; 13: 4209-4212.
- Sharma PK, Kumar M, Vats S. Synthesis and antimicrobial activity of morpholinyl/piperazinylbenzothiazines. Med Chem Res 2012; 21: 2072-2078.
- Fung HB, Kirschenbaum HL, Ojofeitimi BO. Linezolid: an oxazolidinone antimicrobial agent. Clin Ther 2001; 23: 356-391.
- Yoshida K, Nakayama K, Yokomizo Y, Ohtsuka M, Takemura M, Hoshino K, Kanda H, Namba K, Nitanai H, Zhang JZ, Lee VJ. Watkinsc WJ MexAB-OprM specific efflux pump inhibitors in Pseudomonas aeruginosa. Part 6: Exploration of aromatic substituents. Bioorg Med Chem 2006; 14: 8506-8518.
- Bektas H, Ceylan S, Demirbas N, Alpay-Karaoglu S, Sokmen BB. Antimicrobial and antiurease activities of newly synthesized morpholine derivatives containing an azole nucleus. Med Chem Res 2013; 22: 3629-3639.
- Wyrzykiewicz E, Wendzonka M, Kedzia B. Synthesis and antimicrobial activity of new (E)-4-[piperidino (4-methylpiperidino-, morpholino-) N-alkoxy]stilbenes. Eur J Med Chem 2006; 41: 519-525.
- Raparti V, Chitre T, Bothara K, Kumar V, Dangre S, Khachane C, Gore S, Deshmane B. Novel 4-(morpholin-4-yl)-N-(arylidene)benzohydrazides: synthesis, antimycobacterial activity and QSAR investigations. Eur J Med Chem 2009; 44: 3954-3960.
- Kucukguzel SG, Mazi A, Sahin F, Ozturk S, Stables J. Synthesis and biological activities of diflunisal hydrazide-hydrazones. Eur J Med Chem 2003; 38: 1005-1013.
- Reddy PR, Reddy GM, Padmaja A, Padmavathi V, Kondaiah P, Krishna NS. Synthesis, antioxidant, and cytotoxic activities of N-azole substituted thiomorpholine derivatives. Arch Pharm Chem Life Sci 2014; 347: 221-228.
- Avramova P, Danchev N, Buyukliev R, Bogoslovova T. Synthesis, toxicological, and pharmacological assessment of derivatives of 2-aryl-4-(3-arylpropyl) morpholines. Arch Pharm (Weinheim) 1998; 331: 342-346.
- Jakubowska J, Lukawska MW, Czyz M. STI571 and morpholine derivative of doxorubicin collaborate in inhibition of K562 cell proliferation by inducing differentiation and mitochondrial pathway of apoptosis. Eur J Pharmacol 2008; 596: 41-49.
- Piestrzeniewicz MK, Wilmaaska D, Szemraj J, Studzian K, Gniazdowski M. Interactions of novel morpholine and hexamethylene derivatives of anthracycline antibiotics with DNA. Z Naturforsch C 2004; 59: 739-748.
- Shen AY, Hwang MH, Roffler S, Chen CF. Cytotoxicity and antimicrobial activity of some naphthol derivatives. Arch Pharm (Weinheim) 1995; 328: 197-201.
- Alvarez C, Alvarez R, Corchete P, Perez-Melero C, Pelaez R, Medarde M. Synthesis and biological activity of naphthalene anologues of phenstatins: Naphthylphenstatins. Bioorg Med Chem Lett 2007; 17: 3417-3420.
- Matsushita Y, Jang IC, Imai T, Fukushima K, Lee JM, Park HR, Lee SC. Antioxidant and cytotoxic activities of naphthalene derivatives from Diospyros kaki. J Wood Sci 2011; 57: 161-165.
- Budhiraja A, Kadian K, Kaur M, Aggarwall V, Garg A, Sapra S, Nepali K, Suri OP, Dhar KL. Synthesis and biological evaluation of naphthalene, furan and pyrrole based chalcones as cytotoxic and antimicrobial agents. Med Chem Res 2012; 21: 2133-2140.
- Bondock S, Khalifa W, Fadda AA. Synthesis and antimicrobial evaluation of some new thiazole, thiazolidinone and thiazoline derivatives starting from 1-chloro-3, 4-dihydronaphthalene-2-carboxaldehyde. Eur J Med Chem 2007; 42: 948-954.
- Huang MH, Wu SN, Wang JP, Lin CH, Lu SI, Liao LF, Shen AY. Biological study of naphthalene derivatives with antiinflammatory activities. Drug Develop Res 2003; 60: 261-269.
- Rasmussen CR, Villani FJ, Weaner LE, Reynolds BE, Hood AR, Hecker LR, Nortey SO, Hanslin A, Costanzo MJ, Powell ET, Molinari AJ. Improved procedures for the preparation of cycloalkyl-, arylalkyl- and arylthioureas. Synthesis 1988; 456-459.
- Winn W, Allen S, Janda W, Koneman E, Procop G, Schreckenberger P, Woods G. Konemans Color Atlas and textbook of diagnostic microbiology. Lippincott William Wilkins Baltimore Philadelphia (6th edn.) 2006.
- Caliskan F, Ergene E, Sogut I, Hatipoglu I, Basalp A, Sivas H, Kanbak G. Biological assays on the effects of Acra3 peptide from Turkish scorpion Androctonus crassicauda venom on a mouse brain tumor cell line (BC3H1) and production of specific monoclonal antibodies. Toxicon 2013; 76: 350-361.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65: 55-63.
- Sylvester PW. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol Biol 2011; 716: 157-168.
- Fu YJ, Chai BF, Wang W, Zhi H, Yin LT, Liang AH. Expression and purification of the BmK Mm2 neurotoxin from the scorpion Buthus martensii Karsch and its biological activity test. Protein Expr Purif 2004; 38: 45-50.