ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Review Article - Biomedical Research (2019) Volume 30, Issue 4
Soft tissue sarcomas management guidelines
Abdulmalik Altaf1*, Wael Al Shelfa2
1Department of Surgery, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
2Department of Surgery, Zagazig University Hospital, Zagazig, Egypt
- *Corresponding Author:
- Dr. Abdulmalik Altaf
Associate Professor
Department of Surgery
Faculty of Medicine
King Abdulaziz University
Jeddah, Saudi Arabia
E-mail: altaf12345@yahoo.com
Accepted date: June 30, 2019
DOI: 10.35841/biomedicalresearch.30-19-289
Visit for more related articles at Biomedical ResearchSoft tissue sarcomas (STS) are group of rare tumors arising from mesenchymal tissues. It can develop in soft tissues like skeletal muscle, fibrous tissue, fat, nerves, blood vessels, lymphatic vessels, and the deep layers of the skin. The disease can arise in any part of the body, but mostly found in arms and legs, and are also found in internal organs and abdominal cavity. However, soft tissue sarcomas are still uncommon even with more than hundred different types of sarcomas of soft tissues. The disease comprises less than one percent of adult malignancies and twelve percent of pediatric malignancies. As a rare disease with heterogeneity of its subtypes, there is low availability of data to recommend evidencebased management guidelines. STS management guidelines are recent recommendations for surgeons for better diagnosis and treatment of different types of sarcomas, aiming at improving quality of care in patient, and giving them the proper decision for management. This review will summarize clinical presentation, diagnosis, investigation, staging and multidisciplinary treatment of different subtypes of STS based on most recent published guidelines.
Keywords
Soft tissue sarcoma, Guidelines, Management, Staging, Treatment
Introduction
Soft tissue sarcoma (STS) is a relatively rare type of tumor which accounts for less than 1% of adult malignancies [1]. There are more than 100 subtypes of STS which is divided broadly in two main groups, namely soft tissue sarcoma and sarcoma of the bone [2]. Most of the soft tissue sarcomas have no definite cause, and nearly all cases are thought to be de novo rather than malignant transformation of the previous existing benign lesions. However, a number of predisposing factors or risk factors had been incriminated which includes genetic predisposition (neurofibromatosis type I, Le Fraumeni syndrome), chemical carcinogens, chronic irritation, radiation therapy, chemotherapy, lymph edema, and HIV for Kaposi sarcoma [3]. Due to the heterogeneity of subtypes, the history of data for the actual number of incidences is under-reported. According to statistics of the National Cancer Intelligence Network, the number of soft tissue sarcoma incidence is 45 per 1 million populations every year [4]. The incidence of soft tissue sarcoma is higher than bone sarcoma and accounts for about 1/5 of soft tissue sarcoma incidence [5]. Any part of the body can be affected by STS, but it is more common in the extremities. The American College of Surgeon had reviewed the distribution of soft tissue sarcoma in the body parts. They found that most affected body part was the lower extremities (46%), followed by torso (18%), upper extremity (13%), retroperitoneum (13%), and neck and head (9%) [6].
Growth and Spread of Soft Tissue Sarcoma
Soft tissue sarcomas have different grades of growth which depends upon the aggressiveness of the malignant tissue. The growth patterns of tumors are defined as their tendency to grow in the tissue planes, and it rarely invades the bone or fascia. As the tumor grows, it invades the surrounding tissues forming pseudo-capsule. The margins are poorly defined and fingerslike projections of the malignant tumor tissue infiltrate adjacent tissues. Intraoperatively, dissection through pseudo capsule should be avoided; as the residual disease is inevitable. The common mode of spreading the soft tissue sarcoma is through the blood and is mainly spread to the lungs. At the time of diagnosis distant metastases were found in 10% out of which 83% of them are present in the lung [7].
Lymph node spread
Soft tissue sarcoma rarely spread in the regional lymph nodes. A study performed at the Memorial Sloan-Kettering cancer centre, found a total of 46 (2.6%) out of 1772 patients to have regional node metastases at the time of diagnosis [8]. In 2010 the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging system for soft tissue sarcoma, classified the N1 disease as stage III instead of stage IV, regardless of the primary site [9]. In 2017 AJCC/ UICC staging system had been revised again to consider the nodal metastasis as stage IV especially in case of retroperitoneal sarcomas for soft tissue sarcomas arising from extremities and trunk [10].
Recurrence of soft tissue sarcoma
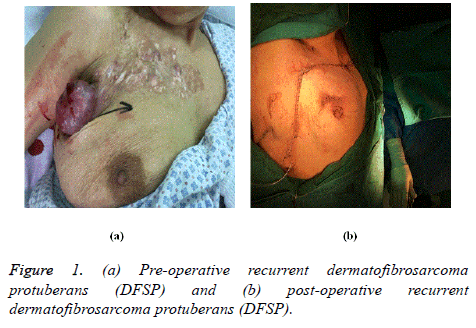
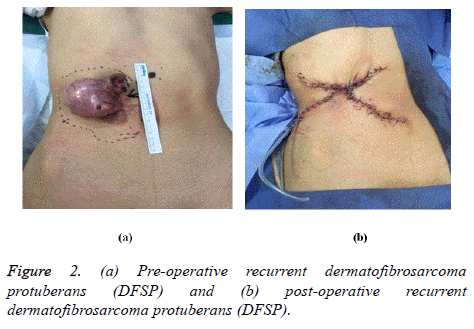
There are two forms of recurrence after soft tissue sarcomas surgeries including both local recurrence and distant metastasis. These types of recurrence are influenced by many factors such as degree of differentiation, histological type, margin and extent of resection, and perioperative radiotherapy [11]. Around 25% of the patients develop recurrence after successful surgery of the primary site. But the incidence of recurrence can increase up to 40% to 50% if the tumor is greater than 5 cm in size or poorly differentiated, deep to fascia [12] (Figures 1 and 2).
Guidelines Objectives
The main objective of the guidelines is to review the data available about soft tissue sarcomas and summarize the recommendations to achieve the best practice and a better quality of management and helps to identify and notify the key decision involved in the management. Further, they also give a valuable resource for sarcoma services for helping and guiding case discussions and patient management with the multidisciplinary team.
Methods of updating guidelines
The updated guidelines were written and reviewed by specialists involved in the diagnosis and management of patients suffering from sarcoma. It includes members of Sarcoma Clinical Reference Group (CRG), British Sarcoma Group (BSG), American National Comprehensive Cancer Network (NCCN), and European Society for Medical Oncology (ESMO). It was developed as a result of consensus expert’s opinion which was based on the understanding of present data and their own clinical experiences.
Scope of guidelines
The guidelines’ recommendations are mainly applied for soft tissue sarcomas arising from limbs and trunk. However, some tumors like Ewing sarcoma and rhabdomyosarcoma require a different approach for management. These rare subtypes are more common in pediatric and young adults [13]. Specific recommendations for the management of retroperitoneal, uterine sarcomas, and desmoid tumors are included separately within this guideline. Gastrointestinal stromal tumors, and bone sarcomas also have its own guidelines [14]. Each centre dealing with sarcoma patients must have a multidisciplinary team. The team should include radiologist, surgeon, expert pathologist, medical oncologist and professional nursing stuff. The surgical team should include surgical oncologist, plastic surgeon, vascular surgeon, thoracic surgeon, and orthopaedic surgeon. Allied health professionals are also included, such as physiotherapists, dietary specialists, supportive, palliative, and home care services in the management. A weekly team meeting for discussing newly suspected cases, and cases under management, with their minutes of the meeting should be provided to referring clinicians.
Clinical Presentations
Soft tissue sarcomas are commonly presented with a gradually enlarging, painless mass of tissue. These masses can be huge, especially in the thigh and retroperitoneum region. Pain and compression symptoms like paresthesia or edema of the extremity may be found in some patients. Rare symptoms include fever and weight loss. Due to the heterogeneity of the site of origin, the clinical features of soft tissue sarcomas are difficult to clearly define. Three symptoms increase the susceptibility of STS including increase in the size of mass being painful, and size of mass greater than 5 cm [15]. A soft tissue mass which keeps on increasing in size should be referred to an ultrasound scan or sarcoma diagnostic centre. In case the ultrasound cannot distinguishes the mass as a benign lesion, then either further investigations are needed, or they are referred to a cancer center. A retroperitoneal or intraabdominal mass maybe considered as STS and they must be referred to the sarcoma centre.
Diagnosis
The initial evaluation of a suspected soft tissue sarcoma included the full history about the time of the start of lesion, rate of growth, expected symptoms of neuro-vascular compression, and/or weight loss. It also includes the physical examination for size, depth, fixation, edema, and nerve compression. Due to painless nature of the disease, the patients generally do not seek medical advice. Thus, delay in the diagnosis of soft tissue sarcoma is very common [16]. The imaging studies are used to determine the nature of the lesion, extent of primary lesion and metastatic lesions, if present. Histopathological examination is essential for diagnosis, and management plan.
Imaging
A suspected patient with soft tissue sarcoma must be immediately referred for undergoing an initial ultrasound scan or must be transferred to a diagnostic centre for triple assessment which included biopsy, imaging and clinical history. Initial ultrasound scan is useful for benign lesions as lipomas. Suspicious ultrasound lesions should have the MRI scan of the affected region. For retroperitoneal tumors, CT scan is more effective and as useful as MRI. Simple X-ray can be used for identifying the calcifications, bone involvement and fracture [17].
Staging
After diagnosis of soft tissue sarcoma, the patient should be staged to exclude distant metastasis, especially in the lungs. A CT of chest should be done to exclude the pulmonary metastasis before the definitive treatment [18]. Simple X-ray of the chest is suitable for elderly, small, and/or low-grade lesion. Abdomen and/or pelvis and isotope bone scanning are not routine staging investigations. Further staging investigations depends upon the histological type and clinical features. Sarcomas with a high risk of nodal metastases include clear cell sarcoma, epithelioid sarcoma and synovial sarcoma, so, CT or MRI is done for regional node assessment [18].
Soft tissue metastases are more common in myxoid liposarcoma therefore pelvic and abdominal CT scan must be done. Whole-body MRI can identify occult metastatic lesions, yet it is not established as a routine investigation [19]. Brain metastases are common in sarcomas such as clear cell sarcoma and alveolar soft part sarcoma therefore, brain CT or MRI is advised [20]. Positron emission tomography scanning (PET scan) is not in use till now as a routine investigation in the case with sarcomas, however, it is considered before radical surgery, or for recurrent cases. Also, it can replace both bone scan and CT scan as a single investigation such as in the case of Ewing sarcoma and Rhabdomyosarcoma [21]. PET is also used in the diagnosis of neurofibromatosis 1 associated malignant peripheral nerve sheath tumors. The tumor ’ s response to systemic treatment can also be assessed by PET scan [22]. The present 8th edition of the American Joint Committee on cancer staging manual (AJCC) suggests the use of chest CT to assess pulmonary metastases [23].
Biopsy
Histologic examination of a soft tissue mass is essential for its diagnosis and treatment plan. If technically feasible, the preferred method for obtaining the tissue is via core needle biopsy. If an incisional biopsy is required, it should be carefully planned and performed by the surgeon who will be doing the definitive resection. A poorly placed initial biopsy may prevent successive surgical resection, preparation of flaps, and/or cosmetic repair, or may require more extensive surgery for encompassing the biopsy site during definitive resection. It is recommended that all the suspected soft tissue sarcomas pathology specimens should be reviewed by the professional pathologist who is specialized in the assessment of soft tissue tumors. Histological diagnosis should be according to the World Health Organization (WHO) 2013 classification, in which stage and grade of the tumors are determined. If possible, the grade must be provided for all the cases [2]. The grading system generally used for tumors in Europe is the Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC), which differentiates it into three grades (Table 1).In the case of round cell liposarcomas of myxoid, there is a different grading system based on round cells percentage and is often used. The imaging studies may provide more information and histology can be revised following the assessment of comprehensive surgical resection of the specimen.
| Tumor differentiation | Necrosis | Mitotic count |
|---|---|---|
| Well | 0:abscent | 1:n˂10 |
| Moderate | 1:˂ 50% | 2:10-19 |
| Poor(anaplastic) | 2:˃50% | 3:˃20 |
Table 1. Histological grading criteria used by FNCLCC [24].
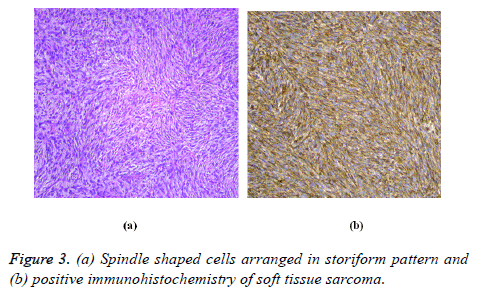
Pathology diagnosis depends on the morphology of cells and immunohistochemistry; however, molecular pathology is needed to confirm the diagnosis of tumors characterized by a specific genetic abnormality (Figure 3). These abnormalities include an activating mutation, chromosomal amplification or chromosomal translocation, use of reverse transcription polymerase chain reaction (RT-PCR) or fluorescent in situ hybridization (FISH). It may be used particularly when there is a doubtful histological diagnosis or clinical pathologic results is unusual. Nowadays the molecular pathology became a routine test to confirm the diagnosis; such as synovial sarcoma, Ewing rhabdomyosarcoma, sarcoma.It is also used to differentiate the lipomas from atypical lipomatous tumors/welldifferentiated liposarcomas [25].
Core needle biopsy, either CT or ultrasound-guided, is considered as a standard methodology for achieving the initial biopsy in most cases due to the low incidence of complications, and high diagnostic accuracy of upto 97.6% of total cases [26]. Incisional biopsy was the historic gold standard method for tissue sampling; however, recently the core needle biopsy became the most common procedure for tissue sampling. Lesions less than 2 cm and superficial to fascia, excision biopsy is preferred. Incision biopsy is preferable than core needle biopsy if definitive diagnosis requires molecular pathology, flow cytometry, or cytogenetics. The larger samples provide the pathologist with more tissue and a better degree of confidence in diagnosis, because of heterogeneity of the tumors.
The surgeon dealing with definitive resection should also perform the incision biopsy because complications or any other bad outcomes may occur when the incision biopsy is done outside the treating centre. The skin incision should be placed longitudinally in the extremity at the time of definitive surgical resection as such the scar can be resected along with the tumor. Further, hemostasis is important to prevent the dissemination of tumor cells [27]. It is not recommended to use fine needle aspiration (FNA) for the initial diagnostic evaluation of a suspected soft tissue sarcoma. It has a lower accuracy than core needle biopsy and does not provide the histologic subtype nor the grade of the sarcoma. However, FNA can be useful in confirming recurrence of the sarcoma [28].
Surgical margins are classified historically into 4 types including radical, wide, marginal, and intralesional, and is still used in the current guidance [29]. The intralesional margin runs through tumor and thus the tumor persists. The marginal surgical plan runs through pseudo capsule, having high local recurrence rate due to tumor satellites. The wide surgical margin is present in the normal tissue but in the same compartment as the tumor. The recurrence rate of wide surgical margin is low. In the case of radical excision, the tumor is removed along with the affected compartments with minimal rate of local recurrence. But the Royal College of Pathologists is recently focusing more on the clearance in millimeters of the closet surgical margin, whether the invasive margin is pushing or infiltrative; type of tissue at the margin (such as skin, muscle, fat or fascia) and presence of vascular invasion. Margin can be simply classified based on the presence and absence of tumor at the cut edge:
R0- no tumor at the cut edge
R1- tumor extends to cut edge
R2- macroscopic residual tumor
Margin assessment is complex and takes into account the type of sarcoma and margin. The planned margin could be positive for tumor tissue intentionally and have a different prognostic significance [25].
Staging
For soft tissue sarcomas, the tumor, node and Metastases or TNM system is the most commonly used staging system. The system was developed as a collaborative effort of the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC). It considers the lymph node involvement (N), tumor size (T), histologic grade (G) and distant metastasis (M) for determining the stage grouping for soft tissue sarcomas. The most recent version of the TNM staging classification (8th edition, 2017) contains separate T staging criteria and prognostic stage groups for soft tissue sarcoma arising in the extremity, trunk and retroperitoneum. Also, there is a unique primary tumor definition for soft tissue sarcoma of the viscera of the abdomen and thorax [30] (Table 2).
| Classification | Description |
|---|---|
| Primary tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor ≤ 5 cm in greatest diameter |
| T1a | Superficial tumor |
| T1b | Deep tumor |
| T2 | Tumor˃5 cm in greater diameter |
| T2a | Superficial tumor |
| T2b | Deep tumor |
| Regional lymph node (N) | |
| NX | Regional LN cannot be assessed |
| N0 | No regional LN metastases |
| N1 | Regional LN metastases |
| Distant metastases (M) | |
| M0 | No distant metastases |
| M1 | Distant metastases |
| Histologic grade (G) | |
| GX | Grade cannot be assessed |
| G1 | Well differentiated |
| G2 | Moderately differentiated |
| G3 | Poorly differentiated |
Table 2. AJCC TNM classification for STS [30].
Stage I
• IA=low grade, small (G1/X, T1a/b, N0, M0)
• IB=low grade, large (G1/X, T2a/b, N0, M0)
Stage II
• IIA=intermediate or high grade, small (G2/3, T1a/b, N0, M0)
• IIB=intermediate grade, large (G2, T2a/b, N0, M0)
Stage III
•High grade, large, (G3, T2a/b, N0, M0)
•Regional node involvement, with any size and grade of the primary tumour (G1-3, T1-2, N1, M0)
Stage IV
•Metastasis identified (G1-3, T1-2, N0-1, M1)
Management of Localized Disease
The major therapeutic goals include the avoidance of local recurrence, long term survival, restoring function and decreasing the morbidity. Decisions about radiotherapy, chemotherapy, surgery and the timing of all the modalities must be made by sarcoma multi-disciplinary team (MDT). In case of limb and trunk tumor its standard treatment i.e., conservative surgery is recommended along with pre-or postoperative radiotherapy. It can help to achieve greater local control rates and maintain the optimal function. However, radiotherapy can be avoided in case of low-grade sarcoma patients whose sarcomas have been completely resected, or in the patient with small superficial high-grade sarcomas which is resected with a wide margin.
Surgery for Localized Disease
The main treatment method for all patients with adult-type localized soft tissue sarcoma is the surgery which should be performed by well-trained surgeon only. The R0 resection is the main aim of surgery which includes with it a margin of normal tissues. The appropriate level of margin of normal tissue included with R0 resection is not accepted universally but 1 cm of soft tissue or equivalent like fascia is commonly acceptable. If without the loss of critical anatomical structure (such as nerve or major blood vessel) the wide resection is not possible then, in that case, it is acceptable to leave a planned microscopic positive surgical margin with the risk of local recurrence. Also, it should be discussed with the concerned patient before the surgery [31].
In case of patients with unplanned positive margin at the time of surgery, re-excision must be performed if an acceptable margin can be attained with tolerable morbidity. Further, the macroscopic residual disease provides with poor prognosis and the local control is most likely to be achieved with the addition of post-operative radiotherapy [32]. The borderline resectable tumors because of their size or position must be treated using neo-adjuvant therapy with radiotherapy or chemotherapy, either systemic or regional. This decision will be taken according to the histology of the tumor, which may be sensitive to systemic treatment, and patient’s general condition [33]. Pre-operative radiotherapy should always be achieved for myxoid liposarcoma, as it is highly sensitive to radiotherapy. Other subtypes are less likely to respond to radiotherapy, so the aim is to devitalize the margins of anticipated margin excision [34].
Surgery with Metastatic Lesion
Surgical excision of the primary tumor remains optional as a palliative resection in patients with metastatic disease in cases with favorable prognosis, local symptoms such as pain, ulceration, bleeding, co-morbidity, histological subtype, and extent of metastasis. However, chemotherapy and/or radiotherapy are still considered more appropriate.
Isolated Limb Perfusion
Isolated limb perfusion may be used pre-operatively to reduce the size of difficult, potentially resectable lesions in the extremity when the limb preservation is possible. The local high dose chemotherapy (melphalan) with hyperthermia and tumor necrosis alpha (TNFα) are restricted to the affected limb with the help of venous and arterial cannulation and a tourniquet [35].
Radiotherapy
The pre and post-operative radiotherapy are used as a standard technique for high-grade and intermediate soft tissue sarcoma. The combination between surgery and radiotherapy helps in the preservation of local control, function, and survival to radical resection [36].
Adjuvant Radiotherapy
The post-operative radiation recommended dose is 60-66 in 1.8-2 Gy fractions. In case of limb sarcomas, a two-phase technique using a shrinking field is generally used; for initial larger volume, 50 Gy is used followed by 10-16 Gy for a smaller volume. The dose can be reduced if the field comprises some critical structures (such as Brachial plexus) [36]. Further, in limb sarcomas cases the preoperative radiation therapy uses a lower dose of 50 Gy and a smaller treatment volume which can cover the preoperative tumor volume instead of postoperative tumor bed. This is found to be linked with an increase in acute postoperative complications as compared to the standard post-operative treatment but also with less late toxicity with equivalent tumor control [37].
If the radiotherapy is used prior to operation there is a slightly higher incidence of post-operative morbidity as well as acute wound healing difficulties. Local free flaps might be advantageous to avoid complications. In the case where wound healing is more likely to create problems, such as groin and axilla, the preoperative radiotherapy is considered as less appropriate. Early surgery is advised in case of rapidly growing tumors, or painful tumors. The preoperative radiotherapy can be particularly proved to be advantageous in case of radiosensitive histological subtype such as myxoid liposarcoma, given the degree of tumor shrinkage which can be achieved [33]. The standard regimen is 50 Gy in case of preoperative radiotherapy, over a period of five weeks. It is followed by the surgery approximately four to six weeks after the completion of radiotherapy. Post-operation, a dose of 10-16 Gy may be given after careful consideration if the tumor margins are positive, however it may result in late toxicity [38].
Curative Radiotherapy
Cases with unresectable tumors because of its location, local invasion, or if resection results in the poor outcome or unacceptable morbidity the radiotherapy is considered as a treatment of choice. The radiotherapy can sometimes provide a durable remission in these cases even though the local recurrence rate is high. The outcome depends on the tumor grade, size, and radiation dose. The doses of over 60 Gy can be used. The lower dose palliative radiotherapy can be used in patients with life-limiting co-morbidities [39].
Adjuvant Chemotherapy
The significance of adjuvant chemotherapy still remains unproven in case of most STS. Although it is currently not viewed as a standard treatment, there is a piece of conflicting evidence for it. It can be used for individual patients with highrisk tumors and potentially chemo-sensitive subtypes based on the benefit which cannot be excluded. Types of chemo sensitive soft tissue sarcomas includes chemo-sensitive STS (synovial sarcoma, desmoplastic small round cell, uterine liomyosarcoma and myxoid cell liposarcoma), moderate chemo-sensitive STS (epitheloid sarcoma, pleomorphic rhabdomyosarcoma, leiomtosarcoma and angiosarcomas), relative chemo-insensitive STS (myxofibrosarcoma, endometrial stromal sarcoma, malignant peripheral nerve sheath tumor, clear cell sarcoma and differentiated liposarcoma) and chemo insensitive STS (alveolar soft part sarcoma and extra skeletal myxoid chondrosarcoma) [40].
Guidelines for Localized STS Management
• Localized soft tissue sarcoma best treatment is surgery.
• Resectable lesions are best treated with a wide excision through normal uninvolved tissue.
• Distance of wide margin is controversial, however with adjuvant radiotherapy, tumor free margin (R0) is adequate.
• If the wide local excision is not possible due to anatomical difficulties, a planned surgical margin with microscopically positive margin and adjuvant radiotherapy for intermediate and high-grade sarcomas are accepted management method of tumor control and for maintaining physical function.
• Adequate margin may necessitate amputation to achieve.
• Neo-adjuvant chemotherapy and/or radiotherapy should be considered for cases with borderline resectable tumors, which depends on the histology of the lesion.
• Combination of surgical resection and radiotherapy (pre or post-operative) for patients with high grade sarcomas, large or margin-positive removed lesions.
• Post-operative dose of radiotherapy is 60-66 Gy.
• Preoperative dose of radiotherapy is 50 Gy.
• Preoperative radiotherapy has a better long-term functional outcome, with a comparable rate of disease control, as compared with post-operative radiotherapy. However, acute post-operative wound complications risk is increased.
• Adjuvant chemotherapy is not routinely used but could be used if local control of the lesion is difficult, or if the metastatic lesion is suspected.
Prognosis of Primary Lesion
Prognosis after treatment of soft tissue sarcoma can be done using a well-established nomogram, which includes size, grade, depth, histopathology, and age. However, some specialist centres made an online calculator. Outcomes improved over the past 20 years. The local recurrence is related to grade, size and margins of excision, and most of these recurrences occur in the first five years. However, a French Sarcoma Study Group found late relapse particularly in case of retroperitoneal or huge soft tissue sarcomas [41].
Standard follow up consists of
• Clinical history
• Imaging studies with the help of MRI or ultrasound in case of clinical suspicion, or if the primary site cannot be examined such as pelvic tumors.
• Chest X-ray with chest CT to investigate any abnormalities.
• Follow up late effects of treatment.
Guidelines for Follow up Primary Lesions
The patients suffering from high or intermediate grade sarcomas are followed up every three to four months for the initial two to three years, then two times every year for upto five years, followed by annual checkups for eight to ten years.
The patients suffering with low-grade sarcomas must be followed up every four to six months for three to five years, followed by annual checkups.
Standard follow up practices includes:
The examination of any new symptoms as soon as reported by the patient.
Clinical examination focused on the local recurrence with imaging follows ups in case of clinical suspicion.
Routine chest X-ray for excluding pulmonary metastases. Constant monitoring to detect late effects of treatments.
Prognosis of Advanced Lesions
The treatment method for metastatic lesions is palliation in almost all cases. Around 50% of high-grade sarcoma cases develop distant metastases and sooner or later dies of disseminated disease. It has a median survival of around twelve months starting from the diagnosis of metastases. But a recent data suggests this survival maybe better in outcomes over the period of time with a median of approximately eighteen months [42]. Managing the advanced lesions is a complex task as palliative treatment depends on the presence or absence of symptoms and probable treatment toxicity. Tumor shrinkage is necessary to control symptoms such as pain, bleeding, obstruction and dyspnea. Prolonged quality of life is achieved in the absence of symptoms by stabilization of disease. Survival in some subtypes of soft tissue sarcomas, such as gastrointestinal stromal disease, can be achieved by the absence of disease progression and not by the degree of response [43]. The published chemotherapy response rates in soft tissue sarcomas vary from 10% to 50%, which depends upon the drug used, patient selection and histological type. A good response to chemotherapy can be achieved if the patient is young as there is an absence of liver metastases, with increased survival time.
The current and future trials are focused on targeted therapy, more specifically it uses genomic profiling with an improved understanding of tumor-genesis and drug activity mechanism. With the better understanding of the immune system, the development of new agents such as the immune checkpoint inhibitors showed great potential in other tumor types. With the rarity of lesion with numerous subtypes, an important challenge in the management of sarcoma is difficulty in performing a large number of trials to gain the ideal and standard randomized evidence. These evidences are required while editing the new treatment recommendations (including recommendations for rare subtypes). It is very important to develop multicenter clinical trials which recruit a large number of patients in it. It should aim at systemic treatment and should be personalized according to the genetics or histology of the individual subtype [44].
Management of Local Recurrence
If local recurrence is found, the patient should be carefully staged as it is often accompanied by metastatic lesions. If no metastatic lesion is found, every attempt must be made for regaining the local control by further surgery with adequate margins and radiotherapy, if not used before. Also, amputation may be needed in some of the cases.
Management of Lung Metastases
For cases with proved lung metastases, metastasectomy is the best option. The metastasectomy must be based on the diseasefree period following the primary surgery, the number of lesions in the lung, absence of other metastases and tumor growth. If there is no significant disease-free interval, full staging using CT or PET scan should be done with repetition at an interval of three months. If there is no appearance of new lesions or if the lesion is operable, surgery is recommended. Radiofrequency or microwave ablation can be considered as another approach. A very targeted form of high-dose hypofractionated radiotherapy known as stereotactic ablative radiotherapy, has emerged as another potential option. But it is still not proved that metastasectomy can improve long-term survival [45].
Management of Extra-Pulmonary Metastases
Most of the extra-pulmonary cases are treated with systemic treatment. In selected cases, cryotherapy, radiofrequency ablation, surgery or radiotherapy can be used for limited metastatic disease for prolonging the remission or reducing symptoms. One of the emerging techniques, the electrochemotherapy may prove to be useful in managing the subcutaneous and refractory dermal metastases in certain tumor subtypes, such as angiosarcoma [46].
Guidelines for Management Advanced STS
• Systemic treatment for most of the advanced soft tissue sarcomas are not curative and the median survival time is twelve to eighteen months. Chemotherapy response varies from 10-50%, which depends on the drugs used, tumor grade, histologic subtype, patient age, and size of tumor.
• Type of decided treatment should be guided by the patient performance status, progression rate, extent of the disease, and potential sensitivity to treatment.
• The first-line of standard treatment is single-agent doxorubicin.
• Ifosfamide is a standard option as second-line therapy, however, it may be used in the case where anthracyclines are contraindicated.
• Doxorubicin and ifosfamide combination are not yet proved itself for improving the survival as compared to first-line single agent doxorubicin. Still, the response rate is higher, and it can be considered for individual patients where there is a chance of improvement of symptoms or help other treatment modalities.
• Additionally, the second-line agents also include trabectedin and combination of gemcitabine and docetaxel. The agent choice depends on patient preference, toxicity profile, and histology.
• Beyond the second-line agents, there is a number of other agents like pazopanib and dacarbazine which can be used depending upon the patient’s fitness and funding limitations.
• Locally recurrent disease should be excised surgically wherever feasible. In case of patients with the oligometastatic disease there are many options like radiotherapy, surgery or ablative therapies, microwave and cryotherapy can be utilized for individual cases, even though there are limited data on their survival benefits.
Summary and Recommendations
• Soft tissue sarcomas are a rare and heterogeneous group of embryologic mesenchymal tissues which contain more than 100 different histological subtypes.
• The pathological diagnosis is done on the basis of immunohistochemistry, histological morphology and occasionally molecular testing.
• Soft tissue sarcomas are most commonly presented as an increasing painless mass in the trunk or extremities.
• The presence of distant metastases is uncommon at the time of presentation, but it is more likely present in deep, large, high-grade sarcoma. A total of 80% of such metastases are present in the lungs.
• The diagnosis of a soft tissue sarcoma is often unsuspected. Partial excision of the tumor before referral may be associated with a higher incidence of distant metastatic disease and the need for an extensive reresection that may impact the functional result. In addition, an inappropriately placed diagnostic biopsy may preclude subsequent surgical resection, flaps preparation, and/or cosmetic repair, or in case of more extensive surgery for encompassing the biopsy site and definitive resection time. Because of these issues, early referral of the patient with suspicious soft tissue mass to a specialized centre with a multidisciplinary sarcoma team is recommended.
• It is recommended to perform magnetic resonance imaging (MRI) of the primary extremity lesion and computed tomography (CT) of primary abdominal, visceral and retroperitoneal lesion.
• A core needle biopsy, if technically feasible is the preferred method for tissue sampling. If an incisional biopsy is required, it should be carefully planned and performed by the surgeon who will be doing the definitive resection. A poorly placed initial biopsy may preclude subsequent surgical resection and/or cosmetic repair and flaps preparation or require more extensive surgery for encompassing the biopsy site at the time of definitive resection. It is recommended that all pathology specimens of suspected soft tissue sarcomas should be reviewed by a pathologist who specialized in the assessment of soft tissue tumors.
• Once the diagnosis of a sarcoma is established, chest imaging is recommended to evaluate the pulmonary metastatic disease. In all the patients, chest CT is used rather than chest radiograph in the patient with a high risk of pulmonary metastases (tumors>5 cm, deep-seated, or intermediate or high grade). CT of the abdomen and pelvis is recommended in round cell/myxoid liposarcomas due to the common presentation of extrapulmonary metastases in the abdomen and retroperitoneum. Imaging of the brain is suggested for patients with angiosarcoma and alveolar soft part sarcoma due to high propensity of these tumors for central nervous system metastases.
• Positron emission tomography is not routinely done in staging evaluation of new cases. PET can differentiate neurofibromatosis and a malignant peripheral nerve sheath tumor.
• Soft tissue sarcomas are staged according to the combined American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC), tumor, node, metastasis (TNM) system which is based on tumor size (T), lymph node involvement (N), distant metastases (M) and histologic grade (G). The most recent edition of AJCC/UICC staging manual (8 edition 2017) has separate T staging criteria, and prognostic stage groups for soft tissue sarcoma arising in the extremity/trunk and retro-peritoneum. In addition, there is a unique primary tumor definition for sarcoma of the viscera of abdomen and thorax but no prognostic stage grouping at that site is available.
• The TNM staging system is not in widespread use for retroperitoneal sarcoma. It does not accounts for disease site or histology i.e., the two major prognostic indicators. AJCC recommends the use of a prognostic nomogram to estimate the likelihood of post-operative survival.
In addition to tumor stage, other prognostic variables include age, site and histological subtype. A nomogram developed by Sloan Kettering Cancer Center (MSKCC) is available online to help in predicting survival and treatment decision making for every single patient.
References
- Siegel RL, Cancer statistics. CA: A Cancer J 2018; 68: 7.
- Fletcher CM. WHO classification of tumors of soft tissue and bone. 4th Ed Lyon: IARC Press 2013.
- Gerrand C. British sarcoma group. UK guidelines for the management of sarcoma. Clin Sarcoma Res 2016; 6:7.
- Soft tissue sarcoma statistics. Cancer Research UK 2016.
- NCIN Bone and soft tissue sarcoma. UK incidence and survival. 2nd Edn 2013; 1-17.
- Lawrence Jr W, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D.Adult soft tissue sarcomas. A pattern of care survey of the American college of surgeon. Ann Surg 2010; 205:349.
- Christie-Large M, James SL, Tiessen L, Davies AM, Grimer RJ. Imaging strategy for detecting lung metastasis at presentation in patients with soft tissue sarcoma. Eur J Cancer 2008; 44:1841.
- Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg 2003; 217: 72.
- American Joint Committee on Cancer. Cancer staging Manual. 7th Ed Springer 2010; 291.
- Pollock RE, Maki RG, Baldini EH. Soft tissue sarcoma of the retrperitoneum. AJCC Cancer Staging Manual 8thEd 2017; 531.
- Vezeridis MP, Moore R, Karakousis CP. Metastatic patterns in soft tissue sarcomas. Arch Surg 1983; 118-915.
- Weitz J, Antonescu CR, Brennan MF. Localized extremity soft tissue sarcoma: improved knowledge with unchanged survival over time. J Clin Oncol 2003; 21: 2719.
- National Institute for Health and Clinical Excellence (NICE), Improving outcomes with children and young people with cancer. 2016.
- Gerrand C, Athanasou N, Brennan B, Grimer R, Judson I, Morland B, Peake D, Seddon B, Whelan J. British Sarcoma Group UK guidelines for the management of bone sarcomas. Clin Sarcoma Res 2016; 6: 7.
- Johnson CJ, Pynsent PB, Grimer RJ. Clinical features of soft tissue sarcomas. Ann R Coll Surg Engl 2001; 83: 203-205.
- Chotel F, Unnithan A, Chandrasekar CR, Parot R, Jeys L, Grimer RJ.Variability in the presentation of synovial sarcoma in children: a plea for greater awareness. J Bone Joint Surg Br 2008; 90: 1090.
- Suspected cancer: recognition and referral NICE Guidance NG12. Section 1 2015;11.
- King DM, Hackbarth DA, Kilian CM, Carrera GF. Soft tissue sarcoma metastasis identified on abdomen and pelvis CT imaging. Clin Ortho Relat Res 2016; 467: 2832-2844.
- Seo SW, Kwon JW, Jang SW, Jang SP, Park YS. Feasibility of whole-body MRI for detecting metastatic Myxoid liposarcoma: a case series. Orthopedics 2017; 34.
- European Sarcoma Networking Group. Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow up. Ann Oncol. 2017; 25: 2-12.
- Roberge D, Vakilian S, Alabed YZ, Turcotte RE, Freeman CR, Hickeson M. PET/CT in initial staging of adult soft tissue sarcoma. Sarcoma 2012; 960-994.
- Quartuccio N, Fox J, Kuk D, Wexler LH, Baldari S, Cistaro A, Schöder H. Pediatric bone sarcoma: diagnostic performance of 18f-FDG PET/CT versus conventional imaging for initial staging and follow-up. Am J Roentgenol 2015; 204: 153-160.
- Pollock RE. Introduction to soft tissue sarcoma. AJCC Cancer Staging Manual 8th Ed Amin MB 2017; 489.
- Lin X, Davion S, Bertsch EC, Omar I, Nayar R, Laskin WB. FNCLCC grading of soft tissue sarcomas on needle core biopsies using surrogate markers. Hum Pathol 2016; 56: 147.
- Royal College of Pathologists Dataset for cancer histopathology reports on soft tissue sarcomas. 3rd Ed 2016.
- Strauss DC, Qureshi YA, Hayes AJ, Thway K, Fisher C, Thomas JM. The role of core needle biopsy in the diagnosis of suspected soft tissue tumors. J Surg Oncol 2010; 102:523.
- www.nccn.org
- Wakely Jr PE, Kneisl JS. Soft tissue aspiration cytopathology. Cancer 2000; 90: 292.
- Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clinic Orthp Rel Res 1980; 153: 106-120.
- Raut CP, Maki RG, Baldini EH. Soft tissue sarcomas of the abdomen and thoracic visceral organs. AJCC cancer staging manual. 8th Amin MB 2017; 517.
- Gerrand CH, Wunder JS, Kandel RA, O’Sullivan B, Catton CN, Bell RS, Griffin AM, Davis AM. Classification of positive margins after resection of soft tissue sarcoma of the limb predicts the risk of local recurrence. J Bone Joint Surg Br 2001; 83: 1149-1155.
- Alektiar KM, Velasco J, Zelefsky MJ, Woodruff JM, Lewis JJ, Brennan MF. Adjuvant radiotherapy for margin-positive high-grade soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 2000: 48: 1051-1058.
- Le Grange F, Cassoni AM, Seddon BM. Tumor volume changes following pre-operative radiotherapy in borderline limb and trunk soft tissue sarcoma. Eur J Surg Oncol 2014: 40: 394-401.
- Roberge D, Skamene T, Nahal A, Turcotte RE, Powell T, Freeman C. Radiological and pathological response following pre-operative radiotherapy for soft tissue sarcoma. Radiother Oncol 2010; 97: 404-407.
- Smith HG, Cartwright J, Wilkinson MJ, Strauss DC, Thomas JM, Hayes AJ. Isolated limb perfusion with melphalan and tumor necrosis factor alpha for melanoma and soft tissue sarcoma. Ann Surg Oncol 2015; 3: 356-361.
- Haas RL, DeLaney TF, O’Sullivan B, Keus RB, Le Pechoux C, Olmi P, Poulsen JP, Seddon B, Wang D. Radiotherapy for management of extremity soft tissue sarcomas. Int J Radiat Oncol Biol Phys 2012; 84: 572-580.
- L Davis AM, O’Sullivan B, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Hammond A, Benk V, Kandel R, Goddard K. Late radiation morbidity following randomization to preoperative versus post-operative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 2005; 75: 48-53.
- Al Yami A, Griffin AM, Ferguson PC, Catton CN, Chung PW, Bell RS, Wunder JS, O'Sullivan B. Positive surgical margins in soft tissue sarcoma treated with preoperative radiation: is a positive boost necessary? Int J Radiat Biol Phys 2010; 77: 1191-1197.
- Andrä C, Rauch J, Li M, Ganswindt U, Belka C, Saleh-Ebrahimi L. Excellent local control and survival after post-operative or definitive radiation therapy for sarcomas of the head and neck. Radiat Oncol 2015; 10: 140.
- Salgado R. Imaging of soft tissue tumors. 3rd Ed Springer 2006; 107-116.
- Jacobs AJ, Michels R, Stein J, Levin AS. Improvement in overall survival from extremity soft tissue sarcoma over twenty years. Sarcoma 2015; 2015.
- Harris SJ, Maruzzo M, Thway K, Al-Muderis O, Jones RL, Miah A, Benson C, Judson IR. Metastatic soft tissue sarcomas, an analysis of systemic therapy and impact on survival. J Clin Oncol 2015; 33.
- Grünwald V, Litière S, Young R, Messiou C, Lia M, Wardelmann E, Van Der Graaf W, Gronchi A, Judson I, STBSG E. Absence of progression, not extent of tumor shrinkage, defines progression in soft tissue sarcoma. Eur J Cancer 2016; 17: 44-51.
- Linch M, Miah AB, Thway K, Judson IR, Benson C. Systemic treatment of soft tissue sarcoma-gold standard and novel therapies. Nat Rev Clin Oncol 2016; 11: 187-202.
- Treasure T, Fiorentino F, Scarci M, Møller H, Utley M. Pulmonary metastasectomy for sarcoma: a systemic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open 2012; 2.
- Benevento R. Angiosarcoma of the beast: a new therapeutic approach? Int J Surg Case Rep 2015; 13: 30-32.