ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 6
Polygonum multiflorum water extract protects against nitric oxide by LPSinduced in RAW 264.7 macrophages
Accepted date: November 23, 2016
Chien-Liang Lin1,2, Shu-Ling Hsieh3, Chih-Chung Wu9, Guan-Cheng Huang4, Wan Leung5, Chining-Ting Lee6, Chine-Hsing Lee7,8* and Chung-Yi Chen2*
1Department of Medical Education and Research, Yuan’s General Hospital, Kaohsiung, Taiwan
2Department of Nutrition and Health Science, School of Medical and Health Sciences, Fooyin University, Kaohsiung, Taiwan
3Department of Seafood Science, National Kaohsiung Marine University, Kaohsiung, Taiwan
4Department of Health-Business Administration, School of Nursing, Fooyin University, Kaohsiung, Taiwan
5Department of Radiology and Nuclear Medicine, Yuan’s General Hospital, Kaohsiung, Taiwan
6Department of Nutrition and Health Sciences, Chang Jung Christian University, Tainan, Taiwan
7Department of Nursing, Min-Hwei Junior College of Health Care Management, Tainan City, Taiwan
8Department and Graduate Institute of Pharmacology, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
9Department of Nutrition and Health Sciences, Chang Jung Christian University, Tainan, Taiwan
- *Corresponding Authors:
- Chine-Hsing Lee
Department and Graduate Institute of Pharmacology
College of Medicine, Kaohsiung Medical University, Department of Nursing
Min-Hwei Junior College of Health Care Management, Taiwan
- Chung-Yi Chen
Department of Nutrition and Health Science
School of Medical and Health Sciences, Fooyin University, Taiwan
Accepted date: November 23, 2016
The purpose of this study was to investigate the effects of Polygonum multiflorum Water Extract (PMWE) on the production of inflammatory mediators in RAW 264.7 mouse macrophages induced by Lipopolysaccharide (LPS). We examined effect of PMWE on the cell viability of RAW 264.7 macrophages cells and investigated anti-inflammatory effect of PMWE by the production of proinflammatory cytokines such as Nitric Oxide (NO). No significant changes have been found in the mouse macrophage cell viability by the PMWE at the concentration of 0.1, 0.5 and 1 mg/ml. Furthermore, PMWE significantly inhibited the production of NO in the LPS-induced macrophages at the concentration of 0.1, 0.5 and 1 mg/ml. In addition, The PMWE inhibited the production of NO more strongly than 2, 3, 5, 4’-Tetrahydroxystilbene-2-O-β-D-Glucoside (THSG), which is well-known as a strong antioxidant agent. These results suggest that PMWE has anti-inflammatory effect through the regulating the production of NO in the LPS-induced macrophages.
Keywords
Polygonum multiflorum water extract, Lipopolysaccharide, RAW 264.7 macrophages cells, Nitric oxide.
Introduction
Nitric Oxide (NO) plays a critical role in a variety of physiological processes including in platelet inhibition, blood pressure homeostasis, neurotransmission, immune responses, and inflammation which is well-known as many pathophysiological conditions in response to the tissue injuries and host defense against the invading microbes [1,2]. Macrophages are the main pro-inflammatory cells responsible for invading pathogens resulted from releasing many proinflammatory molecules, especially the free radical NO. The aberrant release of NO has been reported to cause the amplification of inflammation and the tissue injury [3]. Therefore, the pharmacological interference in NO production is a promising chemotherapeutic strategy to regulate the potentially harmful pro-inflammatory activity of macrophages. Although many anti-inflammatory agents have been investigated [4-8], there still remains a large demand for developing new and effective ones.
A recent study has provided the mechanisms behind the action of natural antioxidants on the inhibition of NO production [9]. Plants are considered to be a good source of natural antioxidant molecules especially vitamins, terpenoids, phenolic compounds, tannins, flavonoids, alkaloids, coumarins, and other metabolites. Therefore, considerable attention has been focused on the use of antioxidants, especially natural antioxidants to inhibit NO production [10,11]. Polygonum multiflorum Thunb, a traditional Chinese herb, has been used in the preparation of herbal medicines in many oriental countries such as China, Japan and Korea for a long time, where dried roots have been well-known as a tonic and an antiaging agent in many remedies in traditional Chinese medicine [12]. Several effective constituents of P. multiflorum Thunb had been reported, including in gallic acid, catechin, stilbene glycoside, anthocyanin and anthraquinone [13]. Recent research indicates that in the ethyl acetate fraction of P. multiflorum Thunb extracts, 2,3,5,4'-tetrahydroxystilbene-2-OBeta- d-Glucoside (THSG), showed strong antioxidant activities [14]. THSG has been reported to reduce peroxidation level of brain in mice with Alzheimer's disease [15] and to down-regulate COX-2 expression and scavenging ROMs resulted in anti-inflammatory effects [16]. However, little information is available about the water extract of P. multiflorum Thunb (PMWE) and its pharmacological activity. Therefore, the aim of present study is to isolate the PMWE and evaluate the antioxidant activity in vitro.
Materials and Methods
Extracts preparation of P. multiflorum Thunb
Dried powders of P. multiflorum Thunb (was purchased by the Phytochemistry Laboratory, Department of Pharmacology, Tongji Medical College, Huazhong University of Science and Technology, China) were extracted with 80 ml distilled water at 100°C for 120 min (fexIKA, Germany) and then centrifuged at 25°C, 4500 rpm for 30 min (Allegra X-15R, Beckman Coulter, USA), the supernatant was collected and the residue was extracted twice again. All the supernatants were combined, evaporated at 40°C and freeze-dried. The obtained powders were weighted and stored at-20°C before use [17].
Cell cultures
RAW 264.7, a mouse macrophage cell line, were purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). Cell were cultured in phenol red free Dulbecco’s modified Eagle medium (DMEM) containing 10% heat-inactivated foetal bovine serum, penicillin (100 U/ml) and streptomycin (100 μg/ml) in a 5% CO2 humidified atmosphere at 37°C.
Measurement of nitric oxide
The concentration of NO was assessed as an indicator of NO production with Griess reaction. Briefly, RAW264.7 macrophages were harvested and seeded in 96-well plates (1 × 105 cells/well) for NO production. After 6 h, the plates were pre-treated with various concentrations of samples for 30 min and incubated with LPS (1 μg/ml) for 24 h. The amount of NO was determined by the nitrite concentration in the cultured RAW264.7 macrophage supernatants with the Griess reagent. Absorbance was measured at 540 nm using a microplate reader; a standard curve of sodium nitrite solution was obtained to determine the nitrite concentration [18]. Inhibition of samples against NO production was calculated using the following formula: NO inhibition (%)=(NO concentration LPS-treated-NO concentration sample-treated)/NO concentration LPS-treated × 100%.
Statistical analysis
Statistical analysis was performed with one way ANOVA followed by a Tukey's test. The data are presented as the means ± S.E.M.
Results and Discussion
Toxicity of water extracts of P. multiflorum Thunb
The toxicities of water extracts of P. multiflorum Thunb (PMWE) in RAW 264.7 macrophages were investigated by the cell viability based on MTT assay. The results showed that 0.1, 0.5, 1, and 2 mg/ml PMWE did not exhibit any inhibition on the cell viability at 12 h and 24 h, respectively. However, 5 mg/ml PMWE significantly inhibit on the cell viability at 12 h and 24 h (Table 1).
| Water extract (mg/ml) | Viability of cells (% of control) | |
|---|---|---|
| 12 h | 24 h | |
| 0.1 | 98.3 ± 9.2 | 95.9 ± 12.1 |
| 0.5 | 104.2 ± 3.4 | 102.1 ± 7.2 |
| 1 | 100.0 ± 4.1 | 114.6 ± 7.7 |
| 2 | 101.3 ± 4.3 | 94.4 ± 7.2 |
| 5 | 89.5± 5.2 | 80.1± 3.2 |
Table 1: Effects of water extracts of P. multiflorum Thunb at different concentrations (mg/ml) in RAW264.7 cells proliferation.
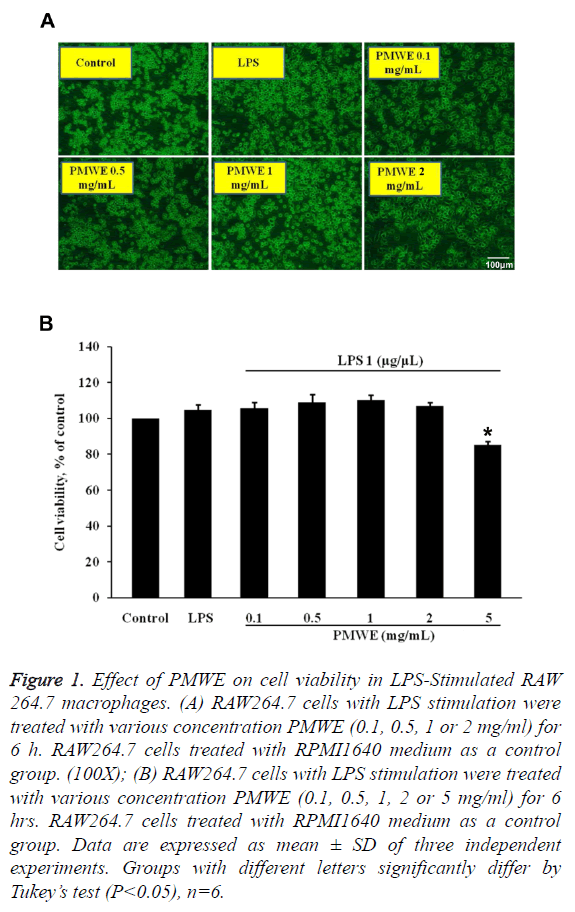
The effect of PMWE on cell viability in LPSstimulated RAW 264.7 macrophages
Firstly, we evaluated the cell viability of RAW264.7 cells with LPS stimulation and PMWE at different dosages. Figure 1 showed that PMWE has no cytotoxicity for LPS-stimulated RAW264.7 cells at various concentrations (0.1, 0.5 or 1 mg/ ml). 2 mg/ml of PMWE cause the morphological alteration of cells, but the cell number did not decrease. On the other hand, PMWE has cytotoxicity for LPS-stimulated RAW264.7 cells at 5 mg/ml. In the present study, we performed the 0.1-1 mg/ml of PMWE was used in the following experiments.
Figure 1: Effect of PMWE on cell viability in LPS-Stimulated RAW 264.7 macrophages. (A) RAW264.7 cells with LPS stimulation were treated with various concentration PMWE (0.1, 0.5, 1 or 2 mg/ml) for 6 h. RAW264.7 cells treated with RPMI1640 medium as a control group. (100X); (B) RAW264.7 cells with LPS stimulation were treated with various concentration PMWE (0.1, 0.5, 1, 2 or 5 mg/ml) for 6 hrs. RAW264.7 cells treated with RPMI1640 medium as a control group. Data are expressed as mean ± SD of three independent experiments. Groups with different letters significantly differ by Tukey’s test (P<0.05), n=6.
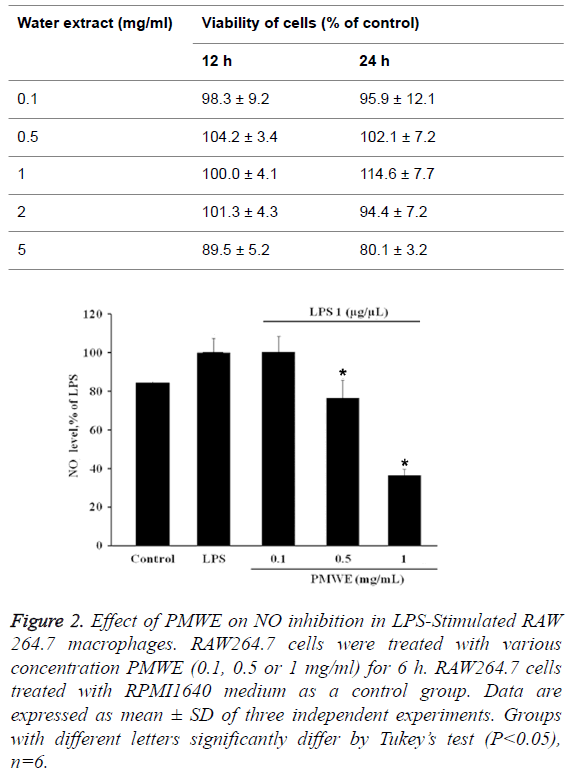
Effects of PMWE on NO production in LPSstimulated RAW 264.7 macrophages
NO is recognized as a mediator and regulator of inflammatory responses. It possesses cytotoxicity properties that are aimed against pathogenic microbes [19]. Pre-treatment with PMWE for 30 min exerted significant repression of LPS-induced NO production in a dose-dependent manner (Figure 2), demonstrating that PMWE significantly suppresses NO production in LPS-stimulated RAW264.7 cells.
Figure 2: Effect of PMWE on NO inhibition in LPS-Stimulated RAW 264.7 macrophages. RAW264.7 cells were treated with various concentration PMWE (0.1, 0.5 or 1 mg/ml) for 6 h. RAW264.7 cells treated with RPMI1640 medium as a control group. Data are expressed as mean ± SD of three independent experiments. Groups with different letters significantly differ by Tukey’s test (P<0.05), n=6.
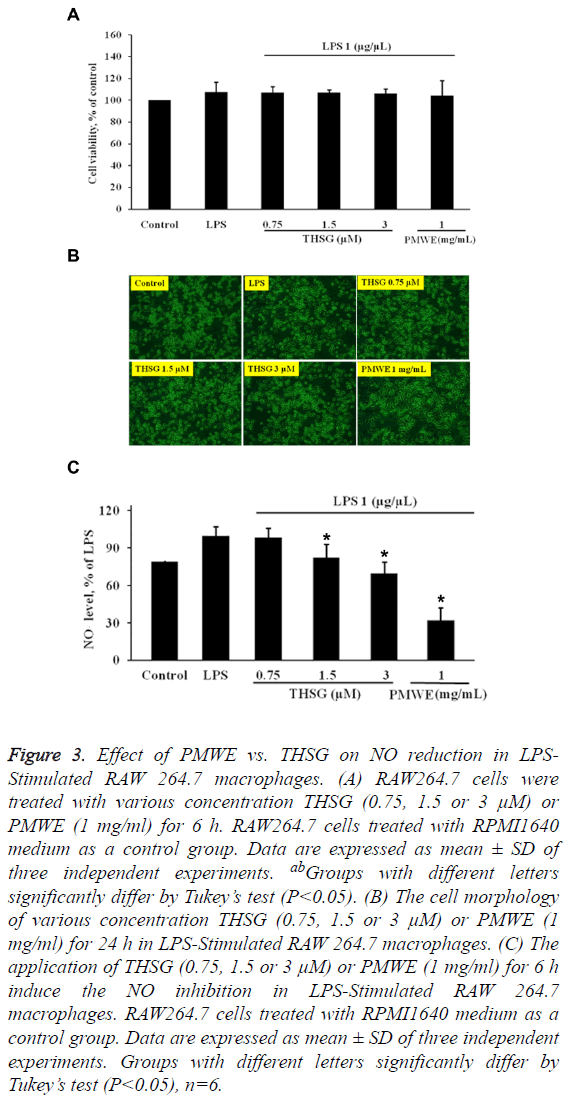
Effect of PMWE vs. THSG on NO reduction in LPSstimulated RAW 264.7 macrophages
THSG, one of the effective components of P. multiflorum Thunb, has been found to attenuate inflammatory responses [15,16,20]. In the present study, PMWE contains 0.2% of THSG. Therefore, 0.5 and 1 mg/ml of PMWE is equal to 1.5 μm and 3 μm of THSG, respectively. The results have shown that THSG has no cytotoxicity for LPS-stimulated RAW264.7 cells at various concentrations (0.7, 1.5 and 3 μm) (Figures 3A and 3B). In addition, PMWE strongly reduced the NO production in LPS-stimulated RAW 264.7 macrophages compared with THSG (Figure 3C), suggesting that the action of PMWE might be superior to that of THSG as a scavenger of oxidative free radicals.
Figure 3: Effect of PMWE vs. THSG on NO reduction in LPSStimulated RAW 264.7 macrophages. (A) RAW264.7 cells were treated with various concentration THSG (0.75, 1.5 or 3 μM) or PMWE (1 mg/ml) for 6 h. RAW264.7 cells treated with RPMI1640 medium as a control group. Data are expressed as mean ± SD of three independent experiments. abGroups with different letters significantly differ by Tukey’s test (P<0.05). (B) The cell morphology of various concentration THSG (0.75, 1.5 or 3 μM) or PMWE (1 mg/ml) for 24 h in LPS-Stimulated RAW 264.7 macrophages. (C) The application of THSG (0.75, 1.5 or 3 μM) or PMWE (1 mg/ml) for 6 h induce the NO inhibition in LPS-Stimulated RAW 264.7 macrophages. RAW264.7 cells treated with RPMI1640 medium as a control group. Data are expressed as mean ± SD of three independent experiments. Groups with different letters significantly differ by Tukey’s test (P<0.05), n=6.
Conclusions
NO is an important molecule for host defense response against various pathogens [21]. It also plays a critical role in the regulation of various pathophysiological processes including neuronal communication, vasodilatation, and neurotoxicity [2,22]. However, overproduction of NO result in tissue damage related with acute and chronic inflammations [11]. Therefore, more attention is now being paid to the development of new drugs as potent inhibitors of NO production to the treatment of chronic inflammatory diseases [23].
Macrophages play important roles in inflammation through the production of several pro-inflammatory molecules, including NO. Production of excessive NO has been associated with a range of inflammatory diseases such as arteriosclerosis, hypertension, ischemic reperfusion and septic shock [11,23]. Several studies have displayed that the plant foods such as fruits, medicinal herbs and vegetables are an excellent source of antioxidant molecules that effectively reduce the inflammatory process by mediating different molecular targets [11,24].
In this study, for the first time, we disclose that the water extracts of P. multiflorum Thunb (PMWE) can suppress NO production in LPS-stimulated RAW 264.7 cells. Meanwhile, PMWE had a strong property to reduce NO production than THSG, which has strong antioxidant activities. We provide evidence to suggest that PMWE might be superior to THSG as a scavenger of oxidative free radicals. Hence, these results suggest that PMWE possesses potential anti-inflammatory activity and holds great promise for the treatment of inflammatory diseases.
Competing Interests
The authors declare that they have no competing interests.
Acknowledgments
This investigation was supported by a grant from Yuan’s General Hospital RG14-012.
References
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J Off Publ Feder Am Soc Exp Biol 1992; 6: 3051-364.
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 1991; 43: 109-142.
- McCartney-Francis N, Allen JB, Mizel DE, Albina JE, Xie QW. Suppression of arthritis by an inhibitor of nitric oxide synthase. J Exp Med 1993; 178: 749-754.
- Liu H, Li Y, Wang XY, Wang B, He HY, Liu JY, Xiang ML, He J, Wu XH, Yang L. Synthesis, preliminary structure-activity relationships, and in vitro biological evaluation of 6-aryl-3-amino-thieno [2, 3-b] pyridine derivatives as potential anti-inflammatory agents. Bioorg Medicinal Chem Lett 2013; 23: 2349-2352.
- Lee SJ, Kim EK, Kim YS, Hwang JW, Lee KH, Choi DK, Kang H, Moon SH, Jeon BT, Park PJ. Purification and characterization of a nitric oxide inhibitory peptide from Ruditapes philippinarum. Food Chem Toxicol Int J Publ Br Industr Biol Res Assoc 2012; 50: 1660-1666.
- Gui X, Wang G, Zhang N, Huang B. New phenylpropanoid and other compounds from Illicium lanceolatum with inhibitory activities against LPS-induced NO production in RAW 264.7 macrophages. Fitoterapia 2014; 95: 51-57.
- Yang L, Wang G, Wang M, Jiang H, Chen L. Indole alkaloids from the roots of Isatis indigotica and their inhibitory effects on nitric oxide production. Fitoterapia 2014; 95: 175-181.
- Kwon J, Basnet S, Lee JW, SeoEK, Tsevegsuren N, Hwang BY, Lee D. Chemical constituents isolated from the Mongolian medicinal plant Sophora alopecuroides L. and their inhibitory effects on LPS-induced nitric oxide production in RAW 264.7 macrophages. Bioorg Med Chem Lett 2015; 25: 3314-3318.
- Yen GC, Duh PD, Huang DW, Hsu CL, Fu TY. Protective effect of pine (Pinus morrisonicola Hay.) needle on LDL oxidation and its anti-inflammatory action by modulation of iNOS and COX-2 expression in LPS-stimulated RAW 264.7 macrophages. Food Chem ToxicolInt J PublBr Indust Biol Res Assoc 2008; 46: 175-185.
- Maksimovia Z, Malencia D, Kovacevia N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour Technol 2005; 96: 873-877.
- Joo T, Sowndhararajan K, Hong S, Lee J, Park SY. Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells by stem bark of Ulmus pumila L. Saudi J Biol Sci 2014; 21: 427-435.
- But PP, Tomlinson B, Lee KL. Hepatitis related to the Chinese medicine Shou-wu-pian manufactured from Polygonum multiflorum. Veter Human Toxicol 1996; 38: 280-282.
- Lin L, Ni B, Lin H, Zhang M, Li X, Yin X, Qu C, Ni J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: a review. J Ethnopharmacol 2015; 159: 158-183.
- Chen Y, Wang M, Rosen RT, Ho CT. 2,2-Diphenyl-1-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum thunb. J Agr Food Chem 1999; 47: 2226-2228.
- Wang X, Zhao L, Han T, Chen S, Wang J. Protective effects of 2,3,5,4-tetrahydroxystilbene-2-O-beta-d-glucoside, an active component of Polygonum multiflorum Thunb, on experimental colitis in mice. Eur J Pharmacol 2008; 578: 339-348.
- Zhang YZ, Shen JF, Xu JY, Xiao JH, Wang JL. Inhibitory effects of 2,3,5,4-tetrahydroxystilbene-2-O-beta-D-glucoside on experimental inflammation and cyclooxygenase 2 activity. J Asian Nat Prod Res 2007; 9: 355-363.
- Lv GP, Meng LZ, Han DQ, Li HY, Zhao J, Li SP. Effect of sample preparation on components and liver toxicity of Polygonum multiflorum. J Pharm Biomed Anal 2015; 109: 105-111.
- Lin CY, Lee CH, Chang YW, Wang HM, Chen CY, Chen YH. Pheophytin a inhibits inflammation via suppression of LPS-induced nitric oxide synthase-2, prostaglandin E2, and interleukin-1beta of macrophages. Int J Mol Sci 2014; 15: 22819-22834.
- Korhonen R, Lahti A, Kankaanranta H, Moilanen E. Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy 2005; 4: 471-479.
- Zhang W, Xu XL, Wang YQ, Wang CH, Zhu WZ. Effects of 2,3,4,5-tetrahydroxystilbene 2-O-beta-D-glucoside on vascular endothelial dysfunction in atherogenic-diet rats. Planta Med 2009; 75: 1209-1214.
- Bogdan C, Rollinghoff M, Diefenbach A. The role of nitric oxide in innate immunity. Immunol Rev 2000; 173: 17-26.
- Nakagawa T, Yokozawa T. Direct scavenging of nitric oxide and superoxide by green tea. Food Chem ToxicolInt J Publ Br Industr Biol Res Assoc 2002; 40: 1745-1750.
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007; 87: 315-424.
- Tsai PJ, Tsai TH, Yu CH, Ho SC. Evaluation of NO-suppressing activity of several Mediterranean culinary spices. Food Chem ToxicolInt J PublBr Indust Biol Res Assoc 2007; 45: 440-447.