ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 16
Neuroprotective and neurorescue effects of eggplant extract in 6-OHDAinduced Parkinson's rat model
Juan Li, Bobo Yuan, Chao Wei and Junxian Gao*
Department of Neurology, Ninth Hospital of Xi’an, Xi’an, PR China
Accepted on July 26, 2017
Objective: Parkinson’s disease (PD) is one of the most common progressive neurodegenerative disorders. Though the exact etiology of PD is largely unknown, oxidative stress has been reported to play an important role in PD. Nowadays, natural products have gained attention as an alternate therapy to delay the onset of neurodegeneration. The aim of the current study was to assess the neuroprotective and neurorescue potential of Eggplant (Solanum melongena L) Extract (EE) in 6-OHDA-induced Parkinsonian rat model.
Materials and Methods: The filtered crude extract of eggplant fruit was prepared and their total flavonoids content was analysed by aluminium chloride (AlCl3) method. Free radical scavenging activity was determined by DPPH assay. Neuroprotective activity was performed on Wistar healthy male models. Phytochemical analysis was carried out by LC/MS analysis.
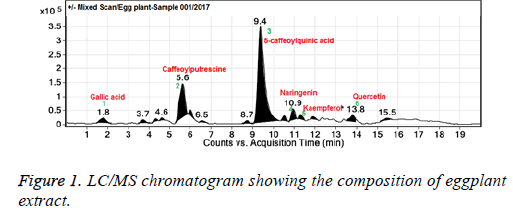
Results: Our results indicated the presence of substantial amounts of phenolics and flavonoids in EE, suggestive of potent free radical scavenging activity. Antioxidant assays revealed that EE exhibits significant ROS scavenging activity. Neurobehavioral analysis showed corrected circling behaviour induced by D-amphetamine and spontaneous locomotor activity in the animals that received EE before or after the 6-OHDA lesioning. Treatment of EE before or after the 6-OHDA lesioning significantly restored the altered levels of oxidative markers in the substantia nigra as well as levels of antioxidant enzymes in the striatum regions of rat brain. Additionally, EE also restored levels of dopamine and dihydroxyphenyl acetic acid and increased expression of tyrosine hydroxylase in the ipsilateral striatum region of the 6-OHDA intoxicated rat brain. Finally LC/MS analysis of the extract revealed the presence of several flavonoids such as kaempferol, quercetin, naringenin and phenolics such as gallic acid, caffeoyl putrescine, and 5-caffeoylquinic acid.
Conclusion: Taken together, our findings indicate that the EE has both neuroprotective as well as neurorescue effects in 6-OHDA-induced Parkinson’s in rat model which could potentially be due to the presence of antioxidant flavonoids and phenolics.
Keywords
Parkinson’s disease, Eggplant extract, Dopamine, Free radical scavenger, Antioxidant, Catalase.
Introduction
Parkinson’s disease (PD) is the most common age related progressive neurodegenerative disorder mainly affecting motor functions. The characteristic symptoms of PD include rigidity, rest tremor, stooped posture and bradykinesia. Although, PD is a multicausal disease, the exact etiology is still largely unknown. The pathological features of PD include degradation of nigrostriatal dopaminergic pathway in the brain along with corresponding decline in the Dopamine (DA) level in striatum, which is believed to be attributed to the combined effect of iron accumulation, glutathione depletion, oxidative DNA damage, elevated lipid peroxidation, excitotoxicity, mitochondrial alterations and attenuated anti-oxidant enzyme activities [1,2]. All these pathological features of PD follow a common cascade of events which is attributed to the oxidative stress [3,4]. Reports have exposed a link between irregular diet habits which lack the required antioxidants like vitamins, foliate and others [5,6]. Deficiency of these antioxidant components increases the level of Reactive Oxygen Species (ROS) further contributing to the onset and progress of the disease [7]. In addition, elevated oxidative stress imparts a major constraint in the L-DOPA treatment, due to autooxidation of L-DOPA and endogenous DA developing quinone and ROS which further accelerate the disease progression [7-9]. Researchers have revealed the role of various antioxidant supplements to overcome oxidative stress mediated consequences which ultimately contribute to neuroprotection [10]. Studies also showed that anti-oxidant supplement along with L-DOPA treatment attenuate oxidative stress associated damage in vitro and as well as in vivo [11].
Currently available PD medications only provide symptomatic relief. No therapeutic intervention is capable to slow down the progress of neurodegeneration in PD. Hence, current research is focusing on finding an alternate therapy, including natural products which could have potential to delay the onset of neurodegeneration in PD [11]. Recently a newer food policy has been projected that particularly reduces the risk of PD in humans and mainly includes nutritional diet with equal amount of food and vegetables and with suitable dietary intake of vitamins [7]. Many food antioxidants like ascorbic acid, selenium, polyphenols, β-carotene and flavonoids found abundantly in fruits and vegetables have shown neuroprotection against oxidative insult through neutralizing ROS [12-15]. Among these dietary antioxidants, phenolics are important beneficiary components for health with numerous biological activities like antiviral, antibacterial, anticancer, anti-inflammatory and hepatoprotective effects [16]. Currently, extensive research has been going on plant and vegetable phenolics due to increasing understanding and awareness about the role of phenolics in human health [17]. Among vegetables, eggplant (Solanum melongena L) is a key source of phenolic and flavonoid compounds both of which are strong free radical scavengers [18]. The major phenolics present in the eggplant include N-caffeoylputrescine, 3-acetyl-5-caffeoylquinic acid and 5-caffeoylquinic acid. Moreover, flavonoids like quercetin and myricetin are also present in its pulp in small quantity [17]. In the present study we evaluated the neuroprotective as well as neurorescue potentials of Eggplant Extract (EE) using 6- OHDA intoxicated Parkinsonian rat model. The current study for the first time reports the neuroprotective effect of EE in 6- OHDA toxicity via scavenging of ROS.
Materials and Methods
Chemicals and reagents
6-hydroxydopamine hydrochloride (6-OHDA), dopamine hydrochloride (DA), thiobarbituric acid (TBA), Damphetamine, trichloroacetic acid (TCA), glutathione reductase (GSH), 3, 4-dihydroxyphenyl acetic acid (DOPAC), mouse anti-TH and mouse anti-β-actin primary antibodies, HRP–conjugated secondary antibody were purchase from Sigma Chemical Co., USA. The ECL Plus chemiluminescent kit was procured from Invitrogen Corporation, USA.
Extract preparation
The eggplant fruits were randomly harvested from the middle of each plant in the field at commercial maturity stage. Healthy and injury free fruits were sorted from the pooled lot. After peeling the eggplant fruits with knife, 20 g of skin and pulp were homogenized using a blander for 2 min in 100 ml double distilled water (ddH2O) (20%). The homogenate was centrifuged for 15 min at 15,000 rpm. Later, the obtained supernatant was vacuum filtered using Whatman-2 filter. The filtered crude extract was then used for further experimentation.
Total phenolics assay
The estimation of total phenolic content was performed following the method. Briefly, 1 ml of ethanol (95%) and 5 ml of ddH2O were mixed with 1 ml of EE. To the mixture 0.5 ml of Folin-Ciocalteu reagent (50% v/v) was incorporated and mixed. After 5 min incubation period, 1 ml of Na2CO3 (5%) was added and resulting mixture was kept aside for 1 h. Lastly, the solution was read spectrophotometrically at 725 nm. For calculation, standard calibration curve was prepared using gallic acid at different concentrations in ethanol (95%). The results were expressed as gallic acid equivalents.
Total flavonoids assay
Total flavonoids content was analysed by aluminium chloride (AlCl3) method [19]. 0.3 ml of NaNO2 (5 %), 4 ml of ddH2O and 0.3 ml of AlCl3 (10%) were mixed with 1 ml of EE. The resulting mixture was incubated at room temperature for 6 min. Later, 2 ml of NaOH (1 M) in the mixture was added and finally adjusted to 10 ml using ddH2O. Immediately the solution was read at 510 nm wavelength using UV spectrophotometer. Quercetin was used to prepare standard calibration curve and the results were expressed as quercetin equivalents.
Phytochemical analysis by LC/MS
The phytochemical fingerprinting of eggplant was carried out by LC-ESI-MS using LC-MS QqQ-6410B (Agilent Technologies) comprising of a chromatographic system (1260 Infinity Agilent Technologies) coupled with an Agilent Triple Quad mass spectrometer fitted with an ESI source. MS conditions were the following: MS range 100-1200 Da, MSn spectra were obtained using both positive and negative modes, gas temperature 325°C, nebulizer gas 45 Psi, capillary voltage 4000 V. HPLC analysis was carried out by an Agilent 1260 infinity series. A Chromolith RP-18e column (4.6 mm ID, 50 mm length) (Merck) was used. The column temperature was maintained at 30°C. The mobile phase consisted of mixture of water and 0.1% FA and acetonitrile. Gradient elution was programmed as follows: 0-10 min, 10-50% B; 10-15 min, 50% B; 15-17 min, 50-100% B and 17-19 min, 100%. The flow rate was optimized to 1 ml/min and the sample injection volume was 5 μl.
DPPH assay
Potential of EE to scavenge the free radicals was determined using DPPH assay. Ethanol (1.5 ml) was mixed with 0.5 ml of EE (100-500 μg/ml). Thereafter, 0.5 ml of DPPH (0.5 mM) solution was added and the mixture was incubated for 10 min at 25ºC. After incubation, the solution was read at 515 nm wavelength using UV spectrophotometer. The percentage radical Scavenging activity (S) of EE was calculated using equation
S=100-(Ax/Ao) × 100
Here Ax and Ao are optical densities of DPPH in the presence and absence of EE respectively.
Animals
Wistar healthy male rats (200-250 g) obtained from the Department of Anatomy and Molecular Histology, Interdisciplinary Graduate School of medicine and Engineering, University of Yamanashi and used for experimental procedures. Animal protocols for the study were approved by the Pathology Department, Ninth Hospital of Xi’an ethical committee. The rats were raised under standard animal house conditions at 12 h light/dark cycle along with food and water ad libitum. During in vivo experiments, 20% EE was prepared daily by mixing 200 g of the entire eggplant fruit with 1 L of drinking water for 5 min in a blander at the end of the afternoon. After filtration, 20% EE was given to the rats ad libitum instead of water. Animals were distributed into the following experimental groups (n=6).
Group I (Sham): Animals were injected with 4 μl of Lascorbate saline (0.2%) (d 0) into the striatum through stereotaxic injection along with drinking water up to 90 d.
Group II (6-OHDA): Animals received 4 μl of 6-OHDA (in L-ascorbate saline (3 μg/μl, 0.2 %)) (d 0) into the striatum through stereotaxic injection along with drinking water up to 90 d.
Group III (EE): Animals were given 20% EE up to 90 d instead of drinking water.
Group IV (Eggplant+6-OHDA): Animals were given 20% EE up to 90 d. The extract was given for initial 45 d. On 46th d, animals underwent 6-OHDA lesioning and the treatment was continued thereafter up to 90 d (neuroprotective assessment).
Group V: (6-OHDA+Eggplant): Animals underwent 6- OHDA injection (d 0) and the treatment of 20% EE was continued from d 1 up to 90 d (neurorescue assessment).
On the 91st d, the neuroprotective and neurorescue potentials of EE were assessed using different neurobehaviour and neurochemical parameters.
Intrastriatal 6-OHDA lesioning
Before performing surgical procedure, anaesthesia was given to the animals using 80 mg/kg, i.p ketamine and 25 mg/kg, i.p. xylazine followed by mounting on a stereotaxic device (Stoelting, USA). A small incision was made to expose the overlying skull. Unilateral striatal dopaminergic neuronal degeneration was developed through injection of 4 μl 6-OHDA (in L-ascorbate saline (3 μg/μl, 0.2 %)) in the striatum: coordinates-lateral 2.5 mm, anterio-posteror 0.5 mm and dorsoventral 4.5 mm with reference to bregma [20]. Moreover, the sham-operated experimental group was injected with equal volume of ascorbate saline. The injection was given at the rate of 1.0 μl/min using an auto injecting pump. Animals were maintained in well ventilated room (25 ± 2°C) in an individual cage till they recovered completely from anaesthesia. Later the three animals per cage were housed together during the experiment. During the first week, food was kept in the cage to facilitate surgical recovery.
Neurobehavioral assessment
Rotational behaviour: Animals from the different experimental groups were subjected to the rotational behaviour. Rats were administered with D-amphetamine (5 mg/kg, i.p.). 30 min after injection and ipsilateral rotations were recorded up to another 30 min [21].
Spontaneous locomotor activity: To assess the spontaneous locomotor activity, rats were individually placed in the photoactometer and the activity counts were measured. Rats were allowed to acclimatize the chamber up to 5 min. Later, the locomotor activity counts were recorded for each rat upto 10 min. The activity counts are the pause in the photo beams which are located parallel in the chamber. After each experiment, chamber was cleaned with 10% ethanol to evade the influence of animal odour, if any.
Neurochemical parameters: After 90 d of treatment, rats were sacrificed to remove the brains immediately to isolate substantia nigra and striatum as per the rat brain atlas [20]. To evaluate the enzymatic antioxidants; super oxide dismutase (SOD) and catalase, striatum was homogenized using phosphate buffer saline pH 7.0 (0.01 M) at a ratio of 10% w/v and centrifuged for 15 min at 15,000 rpm and 4°C to get post mitochondrial supernatant, while substantia nigra was homogenized to estimate the lipid peroxidation and glutathione reductase (GSH) levels.
Lipid peroxidation: To determine the lipid peroxidation, the level of Malondialdehyde (MDA) was determined following the earlier described method [22]. Briefly, the homogenate was transferred into the 0.2 ml eppendorf tube and incubated on water bath for 0 and 1 h at 37°C. After incubation period, 0.4 ml of TCA (5%) and 0.4 ml of TBA (0.67%) were added to both the tubes. The reaction mixture was then centrifuged for 10 min at 10,000 rpm. The resulting supernatant was separated in another eppendorf tube and kept on a boiling water bath for up to 15 min. Finally, the mixture was cooled and read at 535 nm wavelength using UV spectrophotometer. The obtained results were expressed as nmols of MDA formed/min/mg of protein.
Glutathione reductase (GSH): GSH level was estimated according to the earlier described method [23]. Briefly, 0.2 ml of sulfosalicylic acid (4%) was mixed with 0.2 ml of homogenate followed by incubation for 1 h at 4°C. After incubation, the reaction mixture was centrifuged for 15 min at 15,000 rpm at 4°C. Later, 0.1 ml of the supernatant was mixed with 1.7 ml of phosphate buffer saline pH 7.4 (0.1 M) and 0.2 ml of DTNB (0.01 M). The developed yellow color was immediately read spectrophotometrically at 412 nm wavelength. The obtained results are represented as nmol of GSH formed/g of tissue.
Superoxide dismutase activity: Superoxide Dismutase (SOD) activity was determined using the method as described earlier [24]. Briefly, 3 ml of reaction mixture consisted of 0.3 ml of Nitrobluetetrazolium (NBT) (300 μM), 1.2 ml of sodium pyrophosphate pH 8.3 (0.082 M), 0.2 ml of NADH (780 μM) and 0.3 ml of Phenazinemethosulphate (PMS) (186 μM) with 1 ml of striatal tissue homogenate (10% w/v). The reaction was initiated by NADH addition followed by incubation for 90 s at 37°C. Later, addition of 1 ml of glacial acetic acid was done to terminate the reaction. The assay mixture was thoroughly mixed with 4 ml of n-butanol and kept aside for 15 min followed by centrifugation for 15 min at 15,000 rpm. The separated butanol layer was read at 560 nm wavelength to determine the color intensity of formazan. The results obtained are represented as nmol of formazan formed/min/mg of protein.
Catalase activity (CAT): CAT activity was determined using the method as described previously [25] with H2O2 as substrate. Total 1.5 ml of assay mixture consisted of 1 ml of phosphate buffer saline pH 7.0 (0.01 M), 0.4 ml of ddH2O and 0.1 ml of striatal tissue homogenate (10% w/v). Thereafter, 0.5 ml of H2O2 was added to initiate the reaction followed by incubation for 60 s at 37°C. After incubation, the reaction was terminated using 2 ml of glacial acetic acid: dichromate reagent. The reaction tubes were immediately placed for 15 min on boiling water bath and subsequently centrifuged for 15 min at 15,000 rpm. The developed green color was read spectrophotometrically at 570 nm wavelength. The CAT activity was expressed as nmol of H2O2 consumed/min/mg of protein.
DA and DOPAC estimation: At the end of the behavioural experiments, to estimate the striatal DA and DOPAC (DA metabolite) levels, animals from the different experimental groups were sacrificed. Striatum was dissected out, weighed and homogenized using perchloric acid (0.17 M) in a ratio of 10% w/v for 30 s using electric homogenizer to extract the DA and DOPAC from the tissue. The homogenate was kept aside for 15 min for whole protein precipitation. The resulting supernatant was transferred into a separate eppendorf tube and centrifuged for 15 min at 15,000 rpm at 4°C. Later, the resulting supernatant was immediately analysed or stored at -70°C till assayed. DA and DOPAC concentrations were estimated using RP-HPLC system coupled to electrochemical detector (Waters Corporation, USA). Estimation of DA and DOPAC was performed as described earlier with some modifications [26-28]. Briefly, a Sunfire® C18 column (4.6 mm × 150 mm, particle size 5 μm) was used to perform analysis using a mobile phase composed of methanol (15% v/v) in a solution (pH 4.2) of disodium hydrogen orthophosphate (12.5 mM), EDTA (0.5 mM), citric acid (32 mM), KCl (2 mM) and octyl sodium sulphate (0.5 mM) at the flow rate of 1.2 ml/min and operating potential of 0.65 V. The known amounts of DA and DOPAC were spiked in 1 ml of the pooled supernatant to prepare the standard calibration curves which were utilized to determine the DA and DOPAC amounts in the sample by calculating the area under the curve (AUC).
Western blot analysis
Rats were sacrificed from the various experimental groups to dissect the striatum. The tissue lysis buffer consisting of phosphatase and protease inhibitors was used to homogenize the isolated tissue. The homogenates were sonicated for 5 s and centrifuged for 20 min at 15,000 rpm at 4°C. Proteins of same quantity (100 μg) were separated using tris-glycine gel (10%). To block the non-specific binding sites of membrane, it was incubated in 5% non-fat-dried milk for 1 h at 25 ± 2°C and further incubated overnight at 4°C with mouse anti-TH (1:5000) and mouse anti-β-actin (1:5000) primary antibodies. The separated proteins were detected using HRP-conjugated anti-mouse secondary antibody. The proteins were visualized using the ECL kit and were quantified using Image J software.
Statistical analysis
All experiments were carried out in triplicates and expressed as mean ± SEM. The values were considered statistically significant at **p<0.005 and ***p<0.001 was considered statically significant.
Results
Phytochemical analysis
Total phenolic and flavonoid contents are summarized in Table 1. It was observed that 20% EE contained 44.57 ± 2.34 mg/g total phenolic compounds and 5.26 ± 0.42 mg/g total flavonoid compounds. These findings support anti-oxidant activity of EE due to the presence of phenolics and flavonoids. To identify the phenolic and flavonoids present in the eggplant extract we carried out LC/MS analysis of the extract and it was observed the main flavonoid constituents were kaempferol, quercetin, naringenin and the phenolics were gallic acid, caffeoyl putrescine, and 5-caffeoylquinic acid (Figure 1).
DPPH scavenging activity
In the DPPH assay, a stable free radical DPPH gets paired off and reduced by the anti-oxidant into the diphenylpicrylhydrazine. So this assay estimates anti-oxidant ability of a compound via hydrogen atom (or electron) giving activity suggesting its potential to scavenge free radicals. EE exhibited strong antioxidant activity in the DPPH assay with an IC50 of 125 μg/ml (Table 1). Moreover, free radical scavenging activity of EE was found to increase with the increase in the concentration of the extract.
| Total phenolics (mg GAE/g extract) | 44.57 ± 2.34 |
| Total flavonoids (mg CE/g extract) | 5.26 ± 0.42 |
| DPPH scavenging activity (IC50 in µg/ml) | 125 ± 2.15 |
Table 1. Estimation of total phenolics and total flavonoids in eggplant (Solanum melongena L) extract (EE) and its DPPH scavenging activity. GAE: Gallic Acid Equivalents; CE: Catechin Equivalents. Data are expressed as mean ± SEM.
Neuroprotective and neurorescue effects of EE
General observation: At the end of the in vivo experiments, no significant difference was noticed in the body weight among the EE administrated and 6-OHDA lesioned groups compared to the sham-operated animals. Further, the average intake of 20% EE did not significantly change between different treatment groups. Also, average intake of drinking water was found unchanged in the 6-OHDA lesioned and sham-operated groups compared to the 20% EE receiving groups (Table 2).
| Experimental group | Average intake (ml/d) |
|---|---|
| Sham | 24.35 ± 3.42 |
| 6-OHDA | 22.76 ± 2.60 |
| EE | 25.20 ± 3.25 |
| EE+6-OHDA | 23.58 ± 3.47 |
| 6-OHDA+EE | 23.89 ± 3.28 |
Table 2. Average intake of eggplant extracts (EE) or drinking water. Values are represented as mean ± SEM.
Neurobehavioral studies
To check the extent of 6-OHDA-induced unilateral striatal dopaminergic neurodegeneration and to assess the efficacy of EE in attenuating behavioural deficits, we performed different neurobehavioral studies. DA receptor agonist-induced stereotype behaviour was assessed by monitoring unilateral circling behaviour, while as spontaneous locomotor activity was determined by quantifying distance travelled by animals.
EE attenuated circling behaviour induced by Damphetamine
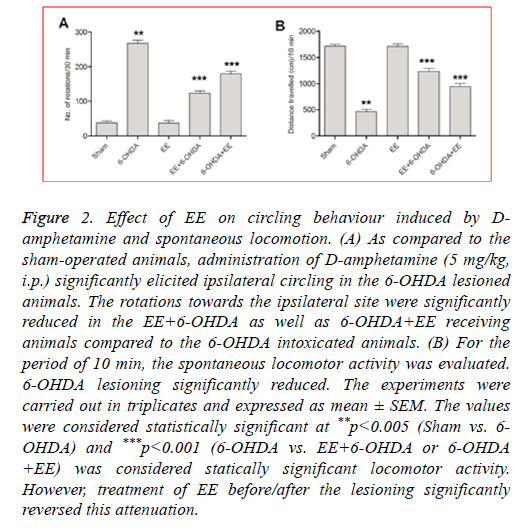
The DA receptor agonist, D-amphetamine induces ipsilateral (lesioned side) circling in the 6-OHDA intoxicated animals. 6- OHDA lesioned animals displayed significantly increased circling behaviour as compared to the sham-operated animals (Figure 2A). As compared to the 6-OHDA group, the rats receiving 20% EE prior to the 6-OHDA lessoning exhibited significantly ameliorated circling behaviour (63%; Figure 2A) whereas 6-OHDA+EE receiving animals exhibited 38% reduction (Figure 2A). No significant difference was seen between alone EE treated group and sham-operated animals. The results showed beneficial effects of EE against Damphetamine- induced stereotypy in 6-OHDA lesioned rats.
Figure 2: Effect of EE on circling behaviour induced by Damphetamine and spontaneous locomotion. (A) As compared to the sham-operated animals, administration of D-amphetamine (5 mg/kg, i.p.) significantly elicited ipsilateral circling in the 6-OHDA lesioned animals. The rotations towards the ipsilateral site were significantly reduced in the EE+6-OHDA as well as 6-OHDA+EE receiving animals compared to the 6-OHDA intoxicated animals. (B) For the period of 10 min, the spontaneous locomotor activity was evaluated. 6-OHDA lesioning significantly reduced. The experiments were carried out in triplicates and expressed as mean ± SEM. The values were considered statistically significant at **p<0.005 (Sham vs. 6- OHDA) and ***p<0.001 (6-OHDA vs. EE+6-OHDA or 6-OHDA +EE) was considered statically significant locomotor activity. However, treatment of EE before/after the lesioning significantly reversed this attenuation.
EE ameliorated spontaneous locomotor activity
To further assess the ability of EE to improve motor alterations, the spontaneous locomotor activity was determined. As shown in Figure 2B, compared to the shamoperated group, the spontaneous locomotion was significantly decreased in 6-OHDA group. Nevertheless, a significant restoration of spontaneous locomotion was observed in the EE +6-OHDA (62%) and 6-OHDA+EE (39%) groups (Figure 2B). No significant impairment of locomotor activity was noticed in the EE alone treated group compared to the sham-operated animals.
Neurochemical studies
6-OHDA lesioning leads to degradation of dopaminergic neurons resulting in significant neurochemical alterations including elevated oxidative stress, reduced DA content and ameliorated Thyroxin Hydroxylase (TH) level. To determine the beneficial effects of EE, recovery from the altered neurochemical changes were evaluated.
Oxidative stress parameters
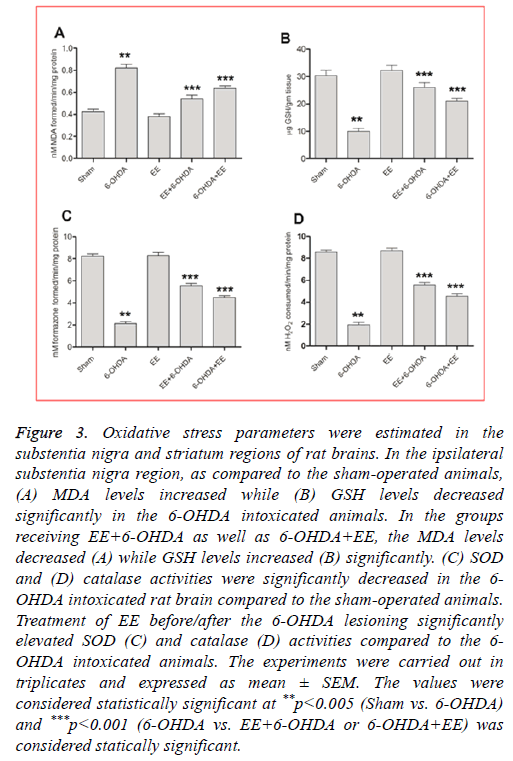
EE corrected lipid peroxidation level: As compared to the sham-operated animals, the lipid peroxidation (MDA) levels were significantly (p<0.001) elevated in the 6-OHDA intoxicated animals (Figure 3A). Rats received EE exhibited significant attenuation in the MDA levels, where as compared to the 6-OHDA group, EE+6-OHDA group showed 70% (Figure 3A) and group receiving 6-OHDA+EE exhibited 47% (Figure 3A) attenuation. The MDA levels were not significantly changed in the group receiving EE alone as compared to the sham-operated animals.
Figure 3: Oxidative stress parameters were estimated in the substentia nigra and striatum regions of rat brains. In the ipsilateral substentia nigra region, as compared to the sham-operated animals, (A) MDA levels increased while (B) GSH levels decreased significantly in the 6-OHDA intoxicated animals. In the groups receiving EE+6-OHDA as well as 6-OHDA+EE, the MDA levels decreased (A) while GSH levels increased (B) significantly. (C) SOD and (D) catalase activities were significantly decreased in the 6- OHDA intoxicated rat brain compared to the sham-operated animals. Treatment of EE before/after the 6-OHDA lesioning significantly elevated SOD (C) and catalase (D) activities compared to the 6- OHDA intoxicated animals. The experiments were carried out in triplicates and expressed as mean ± SEM. The values were considered statistically significant at **p<0.005 (Sham vs. 6-OHDA) and ***p<0.001 (6-OHDA vs. EE+6-OHDA or 6-OHDA+EE) was considered statically significant.
EE restored GSH level: A significant decline in the GSH levels was noticed in the 6-OHDA lesioned animals compared to the sham-operated animals (Figure 3B), which was restored by 79% in EE+6-OHDA receiving animals (Figure 3B) and 54% in 6-OHDA+EE group (Figure 3B) compared to the 6- OHDA intoxicated animals. Nevertheless, no significant alteration was noticed in the GSH level among EE alone treated group and sham-operated group.
EE elevated SOD activity: In the 6-OHDA group, SOD activity was significantly reduced compared to the shamoperated animals (Figure 3C). However, EE treatment significantly corrected this alteration. A significant rise in SOD activity was observed with 56% in EE+6-OHDA receiving animals (Figure 3C) and 38% in 6-OHDA+EE treated animals (Figure 3C, p<0.001). In comparison to the sham-operated animals, alone EE received animals did not showed any significant alteration in SOD activity.
EE corrected catalase activity: The 6-OHDA lesioned group showed significantly reduced catalase activity compared to the sham-operated animals (Figure 3D), which was significantly restored by 55% in the EE+6-OHDA group (Figure 3D) and 39% in the OHDA+EE treated animals (Figure 3D) as compared to the 6-OHDA lesioned animals. The catalase activity did not change significantly in the EE alone receiving animals compared to the sham-operated animals.
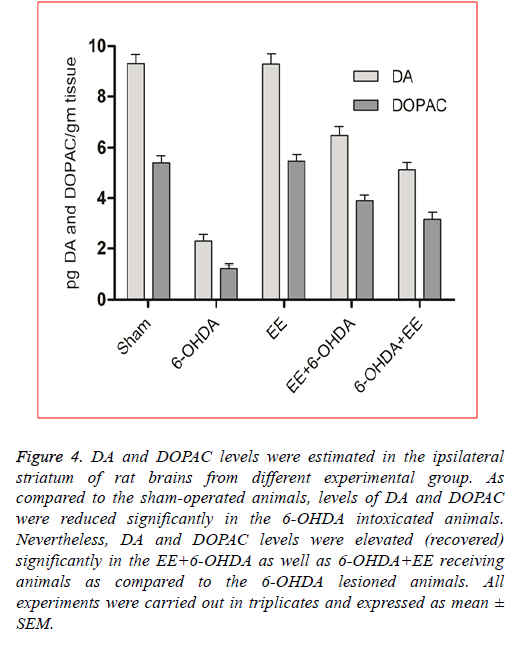
EE restored DA and DOPAC levels: In order to assess the revival of dopaminergic neurons’ functional viability and to show a relationship between neurobehavioral alterations and neurochemical changes, DA and DOPAC levels were assessed in the lesioned site striatum of the rat brains from different experimental groups. As shown in Figure 4, as compared to the sham-operated animals, DA (p<0.001) and DOPAC (p<0.001) levels were significantly reduced in the 6-OHDA intoxicated animals, suggesting significant degradation of dopaminergic neurons in the 6-OHDA lesioned animals. The DA level was restored significantly about 40% in the 6-OHDA+EE group (Figure 4) and 60% in the EE+6-OHDA receiving animals (Figure 4) compared to the 6-OHDA intoxicated group. Moreover, as compared to the 6-OHDA group, DOPAC level was also reversed significantly (p<0.05) by 46% in the 6- OHDA+EE treated animals (Figure 4, and 64% in the EE+6- OHDA receiving animals (Figure 4). Thus, the levels of DA and DOPAC were more prominently and significantly elevated in the EE+6-OHDA receiving animals than the 6-OHDA+EE group as compared to the 6-OHDA lesioned animals indicated improved dopaminergic neurons’ functional viability. Supporting above results, no significant alteration was seen in the DA and DOPAC levels in the EE alone receiving animals as compared to the sham-operated animals.
Figure 4: DA and DOPAC levels were estimated in the ipsilateral striatum of rat brains from different experimental group. As compared to the sham-operated animals, levels of DA and DOPAC were reduced significantly in the 6-OHDA intoxicated animals. Nevertheless, DA and DOPAC levels were elevated (recovered) significantly in the EE+6-OHDA as well as 6-OHDA+EE receiving animals as compared to the 6-OHDA lesioned animals. All experiments were carried out in triplicates and expressed as mean ± SEM.
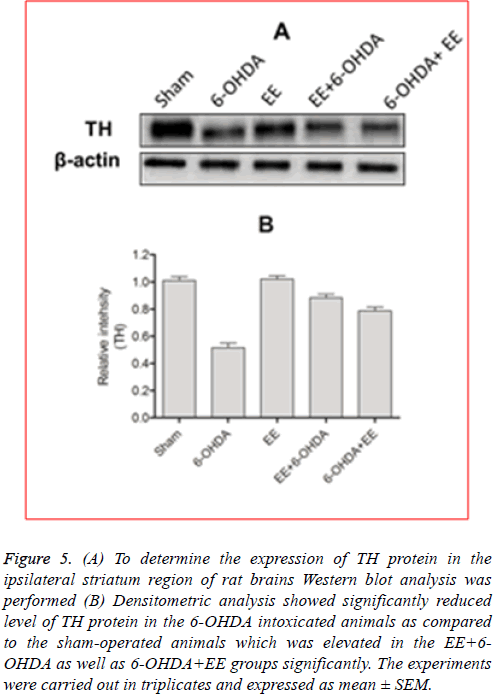
EE restored TH expression: As discussed earlier, the main pathological feature of PD is dopaminergic neurons’ degradation of in the substentia nigra, resulting in decreased level of DA in the striatum. Formation of L-DOPA during the DA biosynthesis is catalysed by TH protein which is considered a rate limiting step in DA biosynthesis. Therefore, PD can be identified as TH-deficiency syndrome [16]. Additionally, to determine the revival of functional viability of dopaminergic neurons, level of TH protein was estimated in the ipsilateral striatal region of the rat brains. Immunoblotting analysis showed that 6-OHDA lesioning ameliorated expression of TH protein significantly in the ipsilateral striatal region, which was significantly restored by EE administration before/after the lesioning (Figure 5A). Densitometric analysis revealed a significant (p<0.001) decrease in the TH protein expression in the 6-OHDA lesioned animals as compared the sham-operated animals (Figure 5B). The level of TH protein observed to be increased significantly (p<0.01) in the EE+6- OHDA (Figure 5B) as well as 6-OHDA+EE (Figure 5B) treated animals in comparison with the 6-OHDA intoxicated animals indicating improved functional viability of the dopaminergic neurons after EE treatment. Experimental group that received EE alone did not exhibit any significant alteration in the level of TH protein compared to the sham-operated animals.
Figure 5: (A) To determine the expression of TH protein in the ipsilateral striatum region of rat brains Western blot analysis was performed (B) Densitometric analysis showed significantly reduced level of TH protein in the 6-OHDA intoxicated animals as compared to the sham-operated animals which was elevated in the EE+6- OHDA as well as 6-OHDA+EE groups significantly. The experiments were carried out in triplicates and expressed as mean ± SEM.
Discussion
The key pathological characteristics of PD involve dopaminergic neuron degradation in the substentia nigra part of brain. Although the etiology of PD is still unknown, oxidative stress is considered as the leading cause of neurodegeneration in PD. Therefore, ROS scavenging anti-oxidants could be effective to combat progressive neurodegeneration induced by oxidative stress in PD. It is a well-recognized fact that phenolic contents in the food play a vital role in imparting health promoting effects because of their antioxidant potential. Natural phenolic compounds possess various biological activities. Studies have also revealed their ability to inhibit proinflammatory cytokines release, and enzymes like lipoxygenase and COX-2 which mediate inflammatory processes [16]. Moreover, advantageous role of flavonoids in the management of diseases conditions like cancer, cardiovascular and neurodegeneration has also been well established which is attributed to their strong antioxidant and radical scavenging effects [29]. Eggplant (Solanum melongena L) is a vital source of phenolic and flavonoid compounds both of which are strong antioxidants [30]. Eggplant is generally known as brinjal, garden egg, aubergine, patlican or melanzana, mostly found in tropical and subtropical regions. The key phenolic components in eggplant are 3-acetyl-5- caffeoylquinic acid, N-caffeoylputrescine and 5-caffeoylquinic acid. Moreover, trace amount of flavonoids, like myricetin and quercetin are also present in its pulp [17]. Among 120 vegetables evaluated for anti-oxidant activity, eggplant ranked among top ten because of its prominent free radical scavenging ability. Eggplant phenolic compounds exhibit angiotensin-I converting enzyme inhibitory activity and α-glucosidase inhibitory activity, suggesting it's possible therapeutic role in the management of type-2 diabetes mellitus and hypertension [31]. Eggplant flavonoids possess potent antioxidant activity against chromosomal aberrations induced by doxorubicin [32]. Previous report also revealed hepatoprotective potential of eggplant in hepG2 cell lie [33]. We have quantified the amount of phenolic and flavonoid compounds. Total phenolic and flavonoid contents were estimated to be 44.57 ± 2.34 mg/g and 5.26 ± 0.42 mg/g of EE respectively. Later, to support these findings, ability of EE to scavenge free radicals was evaluated by means of DPPH assay. EE showed IC50 of 125 μg/ml for DPPH scavenging activity further strengthening its antioxidant potential.
In spite of several findings, the information about neuroprotective as well as neurorescue potentials of EE is lacking. We have demonstrated neuroprotective as well as neurorescue effects of EE against 6-OHDA-induced Parkinsonian rodent model a very first time. 6-OHDA-induced hemi-Parkinsonian rat model is a well-established experimental tool that mimics the neuropathology and neurodegeneration pattern developed during the initial stages of PD. Though a unilateral 6-OHDA injection degrades the striatal dopaminergic neurons, a particular part of the nigrostriatal projections still remains integral, providing ideal conditions to check appropriateness of any neuroprotective agent [34]. Earlier it has been shown that, unilateral 6-OHDA intoxication imparts loss of TH positive dopaminergic neurons almost 60-70% in the striatal region of brain [35].
The 20% EE was administered to the different experimental groups orally before/after the 6-OHDA lesioning. EE intake was not significantly reduced in the groups which received EE in comparison to the animals of sham-operated and 6-OHDA lesioned groups received only drinking water. In the neurobehavioral analysis, 6-OHDA lesioned animals showed increased rotational behaviour induced by D-amphetamine and decreased spontaneous locomotion, suggesting dopaminergic dysfunction and altered motor function, which are closely resemble to PD [34]. D-amphetamine is a DA receptor agonist, which upon administration releases endogenous DA leading to a discrepancy in the levels of DA between unlesioned and lesioned sites of striatum, developing ipsilateral rotations in the 6-OHDA lesioned animals [35,36]. This characteristic behaviour is considered as an important hallmark of nigrostriatal dopaminergic neurodegeneration [34]. In the current study, oral administration of EE before/after lesioning significantly attenuated D-amphetamine-induced rotational behaviour and increased spontaneous locomotor activity, indicating both neuroprotective as well as neurorescue potentials of EE against 6-OHDA insult. The probable mechanism of the neurorescue and neuroprotective effects of EE could be due to presence of prominent and significant amount of polyphenols and flavonoids, which are considered as strong anti-oxidants [18,30]. Previous reports also support the present findings wherein motor deficits have been reported to be attenuated by natural anti-oxidants in Parkinson’s rodent model [13,14].
Previous reports have revealed that altered levels of oxidative stress markers and anti-oxidant enzymes have been well correlated with the 6-OHDA toxicity [13,14,37]. Present findings showed that unilateral 6-OHDA injection increased lipid peroxidation levels and reduced GSH levels significantly in the substentia nigra, by the side of altered anti-oxidant enzyme activities in the striatum including SOD as well as catalase. However, oral intake of EE regulated the altered levels of nigral oxidative markers (lipid peroxidation and GSH) and two key ROS degrading enzymes (catalase and SOD) significantly in the striatal part of rat brain. Moreover, treatment of EE only did not affect the physiological level of these biomarkers of oxidative stress and antioxidant enzyme activity significantly. These beneficial effects of EE may be due to its fundamental properties, i.e. powerful free radical scavenging and anti-oxidant activities [30] and due to presence of antioxidant flavonoids like kaempferol, quercetin, naringenin and the phenolics were gallic acid, caffeoylputrescine, and 5-caffeoylquinic acid as detected by LC/MS analysis. Previous reports have established depletion of DA upon 6-OHDA intoxication in striatum [38,39]. 6-OHDA intoxicated animals showed reduced DA and DOPAC levels which further supported the above findings. In contrast, the DA and DOPAC levels were increased following EE administration significantly. The results revealed potential of EE to attenuate degradation of DA level or probably to reduce the reuptake of DA. The effects could also be attributed to the decrease in auto-oxidation of DA through increased anti-oxidant enzyme activity in striatum and substentia nigra following EE administration. The level of TH protein reduced significantly after 6-OHDA lesioning in the striatum. The treatment of EE, before/after the lesioning, significantly restored the TH protein expression. The results further supported the protective effects of EE on dopamine expressing neurons and restoration of DA. The results are consistent with earlier findings, where neurotoxin mediated dopaminergic neurons’ depletion in the striatum part of brain has been ameliorated by different antioxidants consumed from diet [13,38,39].
Conclusion
The current study revealed neuroprotective as well as neurorescue potentials of EE against 6-OHDA-induced Parkinsonian rat model. It is very early to suggest the molecular mechanism(s) responsible for the experimental neuroprotective effects, our findings anticipated that EEmediated anti-Parkinsonian effect might be attributed to its free radical scavenging, anti-oxidant and DA restoration abilities. If the results obtained from the present study are extrapolated to the humans, consumption of eggplant on regular basis could be useful in ameliorating neurodegeneration and attenuation of the disease progress.
Conflict of Interest
All authors declare that there are no conflicts of interest
References
- Fearnley JM, Lees AJ. Ageing and Parkinsons disease: substantia nigra regional selectivity. Brain 1991; 114: 2283-2301.
- Mosley RL, Benner EJ, Kadiu I, Thomas M, Boska MD, Hasan K, Laurie C, Gendelman HE. Neuroinflammation, oxidative stress, and the pathogenesis of Parkinsons disease. Clin Neurosci Res 2006; 6: 261-281.
- Olanow CW, Jenner P, Youdim M. Neurodegeneration and neuroprotection in Parkinsons disease. Academic Press New York 1996.
- Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Rev 2010; 25: 335-358.
- Agim ZS, Cannon JR. Dietary factors in the etiology of Parkinsons disease. Bio Med Res Int 2015; 672838.
- Duan W, Ladenheim B, Cutler RG, Kruman Ii, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinsons disease. J Neurochem 2002; 80: 101-110.
- Zhu BT. CNS dopamine oxidation and catechol-O-methyltransferase: importance in the etiology, pharmacotherapy, and dietary prevention of Parkinsons disease. Int J Mol Med 2004; 13: 343-353.
- Fahn S, Sulzer D. Neurodegeneration and neuroprotection in Parkinson disease. Neuro Rx 2004; 1: 139-154.
- Foster HD, Hoffer A. The two faces of L-DOPA: Benefits and adverse side effects in the treatment of Encephalitis lethargica, Parkinsons disease, multiple sclerosis and amyotrophic lateral sclerosis. Med Hypotheses 2004; 62: 177-181.
- Mattson MP, DuanW, Chan SL, Cheng A, Haughey N, Gary DS, Guo Z, Lee J, Furukawa K. Neuroprotective and neurorestorative signal transduction mechanisms in brain aging: modification by genes, diet and behavior. Neurobiol Aging 2002; 23: 695-705.
- Koppula S, Kumar H, More SV, Kim BW, Kim IS, Choi DK. Recent advances on the neuroprotective potential of antioxidants in experimental models of Parkinsons disease. Int J Mol Sci 2012; 13: 10608-10629.
- Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MB. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem 1989; 52: 515-520.
- Zafar KS, Siddiqui A, Sayeed I, Ahmad M, Saleem S, Islam F. Protective effect of adenosine in rat model of Parkinsons disease: neurobehavioral and neurochemical evidences. J Chem Neuroanat 2003; 26: 143-151.
- Zafar KS, Siddiqui A, Sayeed I, Ahmad M, Salim S, Islam F. Dose‐dependent protective effect of selenium in rat model of Parkinsons disease: neurobehavioral and neurochemical evidences. J Neurochem 2003; 84: 438-446.
- Simonyi A, Wang Q, Miller RL, Yusof M, Shelat PB, Sun AY, Sun GY. Polyphenols in cerebral ischemia. Mol Neurobiol 2005; 31: 135-147.
- Grussu D, Stewart D, Mcdougall GJ. Berry polyphenols inhibit α-amylase in vitro: identifying active components in rowanberry and raspberry. J Agric Food Chem 2011; 59: 2324-2331.
- Luthria D, Singh AP, Wilson T, Vorsa N, Banuelos GS, Vinyard BT. Influence of conventional and organic agricultural practices on the phenolic content in eggplant pulp: plant-to-plant variation. Food Chem 2010; 121: 406-411.
- Vinson JA, Hao Y, Su X, Zubik L. Phenol antioxidant quantity and quality in foods: vegetables. J Agric Food Chem 1998; 46: 3630-3634.
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999; 64: 555-559.
- Pellegrino LJ, Cushman AJ. Stereotaxic atlas of the rat brain. Appleton Century Crofts New York 1967.
- Shukla S, Agrawal A, Chaturvedi R, Seth K, Srivastava N, Sinha C, Shukla Y, Khanna V, Seth P. Co‐transplantation of carotid body and ventral mesencephalic cells as an alternative approach towards functional restoration in 6‐hydroxydopamine‐lesioned rats: implications for Parkinsons disease. J Neurochem 2004; 91: 274-284.
- Boehme D, Kosecki R, Carson S, Stern F, Marks N. Lipoperoxidation in human and rat brain tissue: developmental and regional studies. Brain Res 1977; 136: 11-21.
- Jollow D, Mitchell J, Zampaglione NA, Gillette J. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974; 11: 151-169.
- Kakkar P, Das B, Viswanathan P. A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys 1984; 21: 130-132.
- Johansson LH, Borg LH. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal Biochem 1988; 174: 331-336.
- Kim C, Speisky MB, Kharouba SN. Rapid and sensitive method for measuring norepinephrine, dopamine, 5-hydroxytryptamine and their major metabolites in rat brain by high-performance liquid chromatography. Differential effect of probenecid, haloperidol and yohimbine on the concentrations of biogenic amines and metabolites in various regions of rat brain. J Chromatogr 1987; 386: 25-35.
- Sharma N, Nehru B. Beneficial effect of vitamin E in rotenone induced model of PD: behavioural, neurochemical and biochemical study. Exp Neurobiol 2013; 22: 214-223.
- Jin G, He XR, Chen LP. The protective effect of ginkobilboa leaves injection on the brain dopamine in the rat model of cerebral ischemia/reperfusion injury. Afr Health Sci 2014; 14: 725-728.
- Kozlowska A, Szostak-Wegierek D. Flavonoids-food sources and health benefits. Rocz Panstw Zakl Hig 2014; 65: 79-85.
- Jung EJ, Bae MS, Jo EK, Jo YH, Lee SC. Antioxidant activity of different parts of eggplant. J Med Plants Res 2011; 5: 4610-4615.
- Kwon YI, Apostolidis E, Shetty K. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour Technol 2008; 99: 2981-2988.
- Sadilova E, Stintzing FC, Carle R. Anthocyanins, colour and antioxidant properties of eggplant (Solanum melongena L.) and violet pepper (Capsicum annuum L.) peel extracts. Z Naturforsch C 2006; 61: 527-535.
- Akanitapichat P, Phraibung K, Nuchklang K, Prompitakkul S. Antioxidant and hepatoprotective activities of five eggplant varieties. Food ChemToxicol 2010; 48: 3017-3021.
- Tieu K. A guide to neurotoxic animal models of Parkinsons disease. Cold Spring Harb Perspect Med 2011; 1: 009316.
- Kirik D, Rosenblad C, Bjorklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol 1998; 152: 259-277.
- Ungerstedt U. Striatal dopamine release after amphetamine or nerve degeneration revealed by rotational behaviour. Acta Physiol Scand 1971; 82: 49-68.
- Ahmad M, Saleem S, Ahmad AS, Yousuf S, Ansari MA, Khan MB, Ishrat T, Chaturvedi RK, Agrawal AK, Islam F. Ginkgo biloba affords dose‐dependent protection against 6‐hydroxydopamine‐induced parkinsonism in rats: neurobehavioural, neurochemical and immunohistochemical evidences. J Neurochem 2005; 93: 94-104.
- LevitesY, Weinreb O, Maor G, Youdim MB, Mandel S. Green tea polyphenol (-)‐epigallocatechin‐3‐gallate prevents N‐methyl‐4‐phenyl‐1, 2, 3, 6‐tetrahydropyridine‐induced dopaminergic neurodegeneration. J Neurochem 2001; 78: 1073-1082.
- Chaturvedi R, Shukla S, Seth K, Chauhan S, Sinha C, Shukla Y, Agrawal A. Neuroprotective and neurorescue effect of black tea extract in 6-hydroxydopamine-lesioned rat model of Parkinsons disease. Neurobiol Dis 2006; 22: 421-434.