ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2011) Volume 22, Issue 1
Mycobacterial secretory SEVA TB ES-31 antigen, a chymotrypsin-like serine protease with lipase activity and drug target potential
Gauri Wankhade1, Anindita Majumdar1, Pranita D Kamble2, BC Harinath1*
1JB Tropical Disease Research Centre, Sevagram, M.S., India
2Department of Biochemistry, MGIMS, Sevagram, M.S., India
- *Corresponding Author:
- B. C. Harinath
JB Tropical Disease Research Centre
Mahatma Gandhi Institute of Medical Sciences
Sevagram-442 102 (Wardha)
M. S. (India)
Accepted date: September 15 2010
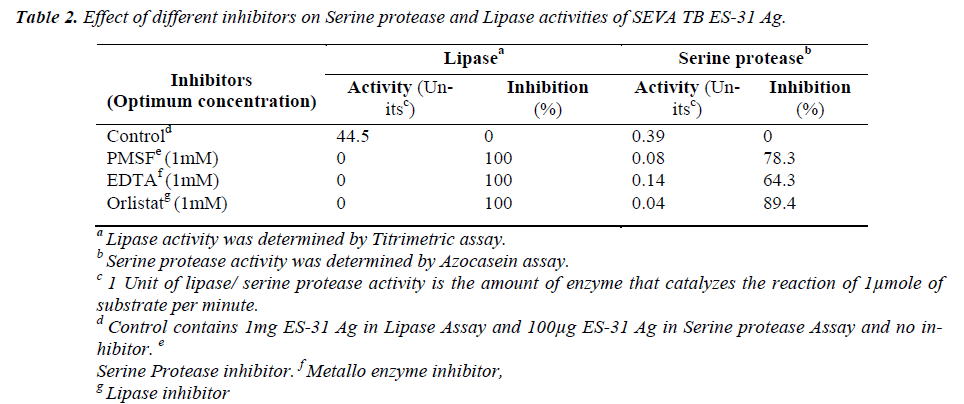
Mycobacterium tuberculosis is known to secrete number of proteins which play an important role in pathogenicity and diagnosis. A diagnostically important secreted antigen, Excretory Secretory-31 (SEVA TB ES-31) protein with serine protease activity was isolated from Mycobacterium tuberculosis H37Ra culture filtrate. Serine proteases like Chymotrypsin have been reported to possess lipase activ-ity as the catalytic triad is similar in serine protease and some lipase enzymes. In this study, ES-31 showed presence of 44.5U/mg pr of lipase activity by titrimetric assay. The lipase activity was inhib-ited by lipase inhibitor, Orlistat by 100%, as well as by serine metalloprotease inhibitors, Phenyl me-thyl sulphonyl fluoride and Ethylene Diamine Tetraacetic acid by 100%. The serine protease activity of ES-31 was inhibited by Phenyl methyl sulphonyl fluoride, Ethylene Diamine Tetraacetic acid, and orlistat by 78.3%, 64.3%, 89.4% respectively. Inhibition of serine protease activity of ES-31 by lipase inhibitor and inhibition of lipase activity of ES-31 by serine protease inhibitor suggests that the cata-lytic site of ES-31 is sensitive to both types of the inhibitors and ES-31 may be a chymotrypsin-like protein with drug target potential
Keywords
Mycobacterium tuberculosis H37Ra, Mycobacterial secreted serine protease, Lipase, Orlistat.
Introduction
Mycobacterium tuberculosis (M.tb.) is an intracellular pathogen, living in the macrophages of host and persists there for a long time. In general, exported proteases in bacteria are associated with bacterial pathogenic viru-lence, however little information is available on M. tb secreted proteins [1]. In our laboratory, SEVA TB Excre-tory Secretory-31 (SEVA TB ES-31), a 31kDa secreted antigen was isolated from culture filtrate of M.tb. H37Ra previously characterized as a serine metallo protease. The proteolytic activity of ES-31 was inhibited by different inhibitors [2,3]. Some lipases share a common Ser-His-Asp/Glu catalytic triad with serine proteases like trypsin, chymotrypsin and subtilisin in their active sites [4,5]. Some of the serine proteases like chymotrypsin and sub-tilisin show lipase activity however not all lipases tested show serine protease activity except commercially avail-able porcine pancreatic lipase (PPL) [5,6].
The present study was carried out to find out the presence of lipase activity in ES-31, as serine protease activity is already determined. Hydrolysis of short chain (water so-luble) esters serves the estimation of esterase activity and that of long chain fatty acids provide the measure of li-pase activity [7]. Therefore olive oil, a substrate of lipase was emulsified in water for the determination of lipase activity by titration. In this study, inhibition of lipase and serine protease activities of ES-31 was assessed using acid-base titration and Azocasein assay respectively.
Materials and Methods
Isolation of tubercular ES-31 serine protease
ES-31 antigen was isolated from M.tb. H37Ra ES antigens by affinity chromatography using anti ES-31 antibody coupled Sepharose-4B column (Pharmacia Biotechnology AB, Uppsala, Sweden) [8]. Briefly, Cyanogen bromide-activated Sepharose 4B beads were coupled with purified anti ES-31 antibody. DSS antigen was passed through column and ES-31 antigen was eluted by glycine HCl buffer (0.01 mol/l, pH2.5) and neutralized with Tris-HCl buffer (0.01M, pH8.6).
Titrimetric Assay
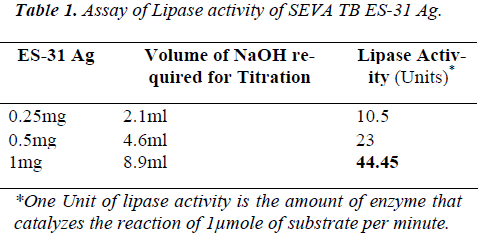
Lipase activity of SEVATB ES-31 antigen was studied at pH 8.5 by titrimetric method using olive oil as a substrate at pH 8.5 at 370C [9]. In brief, an emulsion of substrate containing 2% olive oil with 8mg/ml gum Arabic in 26.7mM Tris-Cl buffer was prepared by sonication. 6.5 ml of substrate emulsion and 1ml of enzyme was incu-bated for 30 min at 370C. Few drops of phenolphthalein indicator solution were added to incubation mixture and titrated till the appearance of light pink color with 5mM NaOH solution. The lipase activity was determined in terms of the amount of NaOH required for the titration. The amount of NaOH required for color change was noted for three different concentrations of ES-31. When Triacylglycerol lipase hydrolyses olive oil, fatty acids are liberated in the substrate solution (pH 8.5) and pH of so-lution decreases. Phenolphthalein turns pink from color-less on the addition of NaOH at pH 8.3. To analyze inhi-bition of lipase activity if any by serine metallo protease inhibitors like Phenyl methyl sulphonyl fluoride (PMSF) (1mM) and Ethylene Diamine Tetraacetic acid (EDTA) (1mM) was checked by titrimetric assay. To find inhibi-tory effect, ES-31 antigen was preincubated at 370C for 1 hour with PMSF (1mM), EDTA (1mM) and Orlistat (1mM)(Commercial Name- Lipocut 60, Manufacturer- Lupin India).
Azocasein Assay
To confirm whether the active site of ES-31 as serine pro-tease and as lipase was the same; inhibition of serine pro-tease activity by lipase inhibitor like Orlistat was checked by azocasein assay. In brief, 5 ml of azocasein assay in-cubation mixture consists of the mycobacterial ES-31 antigen (100 μg) with 25 mg azocasein in 0.5M Sodium Bicarbonate buffer (ph 8.3). The azocasein assay mixture was incubated at 370C for 6 hours. Further, 1ml aliquot solution was removed and 4ml of trichloroacetic acid (5%) was added to the solution. After mixing and filtra-tion using 0.45 μm syringe filters, again 1ml aliquot was removed and 3ml of 500mM NaOH solution was added to the solution. Absorbance of the liberated dye at 440nm was measured using a spectrophotometer (Ultra-spec, Elico Ltd, India) [3]. To find inhibitory effect if any, 100 μg of ES-31 was preincubated at 370C for 1 hr with lipase inhibitor Orlistat (0.5mM, 1mM and 1.5mM), serine met-allo protease inhibitors, phenyl methyl sulphonyl fluoride (PMSF) (1mM) and Ethylene diamine tetraacetic acid (EDTA) (1mM) and assayed for protease activity.
Results
SEVA TB ES-31, a serine protease has shown 44.5U/mg pr of lipase activity by titration. The lipase activity was found to increase in an enzyme concentration dependant manner and there exist a direct correlation between en-zyme activity with enzyme concentration (Table 1).
The lipase activity of ES-31 was inhibited by serine pro-tease inhibitor PMSF (1mM), metalloprotease inhibitor EDTA (1mM) as well as lipase inhibitor Orlistat by 100%. Serine protease activity of ES-31 was inhibited by 1mM EDTA (64.3%) and 1mM PMSF (78.3%) as well as by 1mM Orlistat (89.4%) (Table 2).
Discussion
Some lipases use the same catalytic triad as that of serine protease and also hydrolyze ester bond by the same me-chanism [4]. The catalytic triad (Ser-His-Asp/Glu) com-prises of a Serine residue that has a role in nucleophilic attack on substrate, an acidic amino acid, Aspartate or 48 sometimes Glutamate (in case of lipase) and a Histidine acts as a base [4,5,10].
Serine proteases like chymotrypsin and subtilisin have shown lipase activity [5]. ES-31, a serine protease has also shown lipase activity by titrimetric assay. Lipases hydrolyze triacylglycerol when it is aggregated in an oil-water interface [4,11]. Earlier it was studied that PMSF and EDTA inhibit Bacillus coagulance MTCC-6375 li-pase [7] and ES-31 also inhibited by PMSF and EDTA [3]. To confirm whether the active site of ES-31 serine protease and ES-31 lipase is the same; its inhibition of serine protease activity by lipase inhibitor like Orlistat was checked by azocasein assay and inhibition of lipase activity by serine metallo protease inhibitors like PMSF and EDTA was checked by titration. Orlistat binds to the active site of pancreatic lipase, which leads to some con-formational changes and abolishes its activity [12]. PMSF specifically binds to the serine residue of active site of serine protease and other enzymes having serine in its active site [13], EDTA binds to metal ion required by me-talloproteases and inactivates them [13]. Orlistat inhibited the serine protease activity and PMSF and EDTA inhib-ited lipase activity suggesting that the active site of ES-31 for serine protease and lipase may be same consisting of a typical catalytic triad of Ser-His-Asp. ES-31 may be a chymotrypsin-like enzyme which needs further investiga-tion.
From this study it was found that ES-31 is highly sensi-tive to PMSF, EDTA, and Orlistat. The inhibition of ser-ine protease activity by Orlistat and inhibition of lipase activity by PMSF and EDTA suggests the presence of same catalytic triad responsible for both serine protease and lipase activity of ES-31 Antigen. Earlier studies have shown that ES-31 (Serine protease) has potential to be a drug target [3]. Present study has shown that ES-31 is highly sensitive to a commercial anti-obesity drug Orlistat for both serine protease and lipase activities and thus orl-istat can also be used to inhibit the action of ES-31 Ag. Thus Serine protease and lipase inhibitors or their ana-logues may act as drugs and inhibit the growth of tubercle bacilli.
Acknowledgements
This study was supported by a core research grant of Kas-turba Health society. Thanks are due to Shri Dhiru S Mehta, President, KHS and Dr S. Chhabra, Dean, MGIMS for keen interest and encouragement. Technical assistance of Mrs S. Ingole is appreciated.
References
- Dave JA, Gey van Pittius NC, Beyers AD, Mario RW, and Brown GD. Mycosin-1, a subtilisin-like serine pro-tease of Mycobacterium tuberculosis, is cell wall-associated and expressed during infection of macro-phages. BMC Microbiol. 2002; 2: 30.
- Nair ER, Banerjee S, Kumar S, Reddy MVR, Harinath BC. Purification and characterization of a 31 kDa My-cobacterial Excretory-Secretory Antigenic Protein with a Diagnostic Potential in Pulmonary Tuberculosis. Ind J Chest Dis Allied Sci. 2001; 43: 81-90.
- Upadhye V, Majumdar A, Gomashe A,Joshi D, Gan-gane N, Thamke D, Mendiratta D, Harinath BC. Inhibi-tion of Mycobacterium tuberculosis secretory serine protease blocks bacterial multiplication both in axenic culture and in human macrophages. Scand J Infect Dis 2009; 41: 569-76.
- Brady L, Brzozowski AM, Derewenda ZS, Dodson E, Norskov L, et al. A serine protease triad forms the cata-lytic centre of a triacylglycerol lipase. Nature 1990; 343: 767–770.
- Fujii R, NakagawaY, Hiratake J, Sogabe A and Sakata A. Directed evolution of Pseudomonas aeruginosa li-pase for improved amide-hydrolyzing activity. Protein emerging design and selection 2005; 18: 93-101. 6. Tatsuo M, Mitsutoshi N, Hidemasa K, Kosei K, Minoru S and Masahiro G. Can lipases hydrolyze a peptide bond? Enzyme and Microbial Technology, 2003; 32: 655-657.
- Kanwar SS, Kaushal RK, Jawed A, Gupta R, Chimni SS. Methods for inhibition of residual lipase activity in colorimetric assay: A comparative study. Ind J Bio-chem Biophysics 2005; 42: 233-237.
- Nair ER, Banerjee S, Kumar S, Reddy MVR, Harinath BC. Isolation of Mycobacterium tuberculosis 31 kDa antigen protein of diagnostic interest from culture fil-trate using anti ES-31 antibody by affinity chromatog-raphy. Ind J Clin Biochem 2001; 16: 132-135.
- Choi SJ, Hwang JM, Kim SI. A Colorimetric Mi-croplate Assay Method for High Throughput Analysis of Lipase Activity. J Biochem Mol Biol 2003; 36: 417-420.
- Neuvonen H. Enzyme-substrate interaction in the cata-lytic triad of serine proteases: increase in the pKa of Asp102. Biochem. J. 1997; 322: 351-352.
- Fojan P, Jonson PH, Petersen MTN, Petersen SB. What distinguishes an esterase from a lipase: A novel struc-tural approach. Biochimie 2000; 82: 1033-1041.
- Suwailem AK, Tamimi AS, Omar MA and Suhibani MS. Safety and Mechanism of Action of Orlistat (Tet-rahydrolipstatin) as the First Local Antiobesity Drug-Journal of Applied Sciences Research 2006; 2: 205-208.
- Protease Inhibitors for Protein Isolation: PMSF, AEBSF, Benzamidine. In: BIOSYNTH CHEMISTRY AND BIOLOGY. Available via: http://www.biosynth.com/index.asp?topic_id=194&g=19&m=226.