ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 18
Metabolism of caffeine in non-insulin dependent diabetic patients by physiologically based pharmacokinetic modelling
Introduction: In vitro and earlier animal studies implicated altered metabolism of caffeine as a probe for CYP1A2 enzyme activity in diabetic disease state. However clinical studies in humans reported controversial findings. This study is aimed to compare the differences in the metabolism of caffeine in Non-Insulin Dependent Diabetes Mellitus (NIDDM) patients and in healthy subjects by using Physiologically Based Pharmacokinetic (PBPK) modelling with SimCYP simulator.
Methodology: A mechanistic Physiologically Based Pharmacokinetic (PBPK) model based disposition study for caffeine in healthy subjects and in diabetic patients using SimCYP simulator was performed by altering the CYP1A2 abundance as reported earlier. Prior to using the model for predictions in diabetic patients, model was validated for accuracy by comparing the model predicted and observed pharmacokinetics of caffeine from four independent clinical trials in healthy subjects.
Results and Conclusions: Results from the study confirmed the accuracy of the default SimCYP model for predicting caffeine pharmacokinetics in healthy Caucasian subjects. PBPK model for NIDDM patients found that Caucasian diabetic patient’s caffeine clearance is 37% higher in comparison with healthy subjects and there by reduced exposure of the drug in diabetic patients suggesting a similar fate for other CYP1A2 substrates. Simulations also suggested a clinical trial design that could be utilized to further study the real differences in caffeine disposition in diabetic patients in comparison to healthy subjects.
Keywords
PBPK modeling, Caffeine, Diabetes type II, CYP1A2, SimCYP, Non-insulin dependent diabetes
Introduction
Non-Insulin Dependent Diabetes Mellitus (NIDDM) is highly prevalent worldwide. Moreover, number of patients with NIDDM is increasing with time [1-3]. NIDDM patients are subjected to vascular changes attributed mainly due to hyperglycemic states and are prone to cardio-vascular complications [4]. NIDDM can potentially cause alterations in the disposition and metabolism of xenobiotics [5,6].
Caffeine apart from being constituent of beverages is also commonly used as probe in clinical trials to determine the enzyme activity of CYP1A2 enzyme [7]. Xenobiotics which are CYP1A2 substrates are clinically important drug candidates as they are used in important ailments such as cardiovascular, central nervous system diseases [8-10] and include drugs with narrow therapeutic window [11]. Data on caffeine metabolism is handy for researchers to understand the pharmacokinetic variations of other CYP1A2 substrates.
Disposition of drugs in living organisms is a summation of drug absorption, distribution, metabolism and elimination, alterations in anyone or more eventually effects the overall drug exposure [12,13]. Physiological status of an individual is one of the major contributors for differences in the drug disposition [14]. In diabetes, physiological alterations are noted which perhaps can account for the alterations in the disposition of xenobiotics [5,6]. Fortunately, numerous physiological data is made available and is being used in mathematical modeling of drugs and their disposition. Physiologically Based Pharmacokinetic (PBPK) modeling can be used to predict pharmacokinetics of drugs based on the physiological parameters. PBPK utilizes the wealth of the data on physiological parameters of an individual known as “System data” and the drug data known as “compound data”, which can be as little as physio-chemical properties in some cases to predict the drug disposition [15].
SimCYP simulator is a handy tool which incorporates mechanistic PBPK modeling to predict the deposition of xenobiotics in different scenarios for instance in patients with altered physiology or drugs co-administered with other drugs [16].
Caffeine metabolism in diabetic animal models and in humans reported to be the subject of controversy. Caffeine metabolism in diabetic models also reported to be species depended. Out of 4 studies which specifically studied CYP1A2 activities in diabetic rat model [17-22], 3 reported that there is an upregulation of CYP1A2 enzyme in diabetic rats in comparison to control. Whereas such effects were not noted in diabetic mice models [23].
So far there have been 3 clinical trials [24-26] in humans to reflect on the caffeine metabolism in diabetic patients in comparison to control healthy subjects. Bechtal et al. and Matzke et al. reported increase in caffeine clearance in diabetic patients [25,26]. Whereas Zysset et al. reported no differences in caffeine metabolism between NIDDM patients and healthy subjects [24].
This study reports the simulation results on the caffeine pharmacokinetics in diabetic patients in comparison to non-diabetic patients in relatively large sample size which is not feasible in real clinical trials [27]. SimCYP simulator was used to predict the pharmacokinetics of caffeine in diabetic patients by a model based on the altered CYP1A2 activity in diabetic patients.
Resources and Methodology
This study was performed in two phases. Phase 1, validation of PBPK model for caffeine, default PBPK model for caffeine from SimCYP simulator V 14.0.93 (SimCYP Limited, Sheffield, UK) was utilized by using caffeine compound data from the Table 1 and compared with the real-time caffeine disposition from four clinical trials [28-31]. Phase 2, application of the PBPK model to NIDDM patients, validated PBPK model from phase 1 of the study for caffeine disposition was used to stimulate the disposition of caffeine in virtual populations comprising of 2 groups, Group 1 non-diabetic healthy and group 2 diabetic patients. For group 2 diabetic patients CYP1A2 abundance in SimCyp was adjusted to reflect 42% rise in activity as reported previously by Matzke et al. [25]. Study power was also estimated in the range of 5-500 subjects/patients in each group by setting the statistical significance level to less than 0.01 (p<0.01).
| Parameter | Value |
|---|---|
| Molecular weight* | 194.19 |
| Log P* | -0.07 |
| Pka (at 25°C)^ | 1.05 |
| Water solubility* | 21.6 mg/ml |
| Blood plasma partition coefficient (B/P)^ | 0.977 |
| Fraction unbound (Fu)^ | 0.68 |
| Caco-2 permeability (pH-Apical: Basal (7.4:7.4)^ | 30.8 |
| Km^ | |
| CYP1A2 | |
| N1-demethylation | 157 |
| N3-demethylation | 300 |
| N7-demethylation | 245 |
| Dehydroxylation (-OH) | 265 |
| CYP2E1 | |
| N1-demethylation | 1411 |
| N7-demethylation | 823 |
| Dehydroxylation (-OH) | 1019 |
| CYP3A4 | |
| Dehydroxylation (-OH) | 45080 |
| Vmax^ | |
| CYP1A2 | |
| N1-demethylation | 0.56 |
| N3-demethylation | 13.6 |
| N7-demethylation | 0.21 |
| Dehydroxylation (-OH) | 0.36 |
| CYP2E1 | |
| N1-demethylation | 0.03 |
| N7-demethylation | 0.02 |
| Dehydroxylation (-OH) | 0.18 |
| CYP3A4 | |
| Dehydroxylation (-OH) | 1.8 |
| *Input data obtained from Pubchem open chemistry database. ^Default Input data from compound file for caffeine from SimCYP Simulator v14.1. | |
Table 1: Caffeine compound data used in SimCYP input.
Phase 1 of the study
Validation of PBPK model for caffeine in healthy subject: The compound data for SimCYP input for caffeine was partially obtained from the “Pubchem open chemistry database” and some of the parameters used as default values as in the compound file. Table 1 summarizes the SimCYP input data used.
Advance, Dissolution, Absorption, and Metabolism (ADAM) model in SimCYP to predict absorption and enzyme kinetics to predict the metabolism of caffeine with the contribution of different cytochrome P450 (CYP P450) enzyme was utilized from the default SimCYP compound library for caffeine, Table 1. Minimum PBPK model was implemented and the volume of distribution at steady state was estimated by Rodgers et al. method [32] (SimCYP Method 2).
PBPK model was then validated against the four clinical trials performed in healthy human subjects. Trail design for the SimCYP simulations were matched with a clinical trial performed in healthy volunteers by matching the trial particulars mentioned in Table 2.
| Perera et al. [31] | Liu et al. [30] | Cysneiros et al. [29] | Hammani et al. [28] | |
|---|---|---|---|---|
| No. of subjects | 30 | 30 | 12 | 24 Healthy |
| Age range (y) | 18-50 | 21-54 | 18-35 | 18-50 |
| Caffeine dose (mg) | 100 | 130 | 250 | 300 mg |
| Route of administration | Oral | Oral | Oral | Oral |
| Fasted/unfasted | Fasted | Fasted | Unfasted | Fasted |
| Ratio of females to males | 0 (all males) | 2 | 0.5 | 0.12 |
| Trial duration (h) | 24 | 10 | 24 | 14 |
Table 2: Trial design used to simulate caffeine disposition with SimCYP simulations.
Phase 2 of the study
PBPK model application to simulate caffeine disposition in diabetic patients: Disposition of single oral dose of 250 mg of caffeine in two groups, healthy male subjects and another created group termed as “NIDDM patients” was simulated in SymCyp by using inputs mentioned in Table 1. Study power was estimated to pick a difference in AUC0-24 of caffeine using multiple populations. Ratio for females to males set to “0.5” to indicate equal number of males and females in each group. Only difference in two groups of patients was the liver abundance of CYP1A2 enzyme. NIDDM patients were set to have CYP1A2 abundance value at “74” to reflect the 42% enhanced activity in comparison to non-diabetic patients as reported earlier [25]. For power calculations minimum and maximum patients were set to 5-500 with 20 steps.
Pharmacokinetic calculations
Individual pharmacokinetic calculations for caffeine in healthy subjects and in the diabetic patients groups are calculated by non-compartment analysis and were obtained as the simulation outputs with SimCYP.
Statistical analysis
All the statistical analysis was performed in R package. Test for normality for each PK parameter was performed visually by plotting histograms, box plots and Quantile-Quantile (QQ) further tested by Shapiro test and Anderson darling test (additional R package “nortest” installed). Parameters with the p>0.05 for both Shapiro and Anderson darling test were regarded to reject the null-hypothesis of normal distribution of respective parameter. Parameters failed to show normal distribution were log transformed and subjected to normality test again. Even after the log transformation if parameters failed to pass the test for normal distribution, differences in the medians were compared non-parametrically using Mann- Whitney U test for statistical significance level of “0.05”.
Results and Discussion
Phase 1 study, PBPK model validation
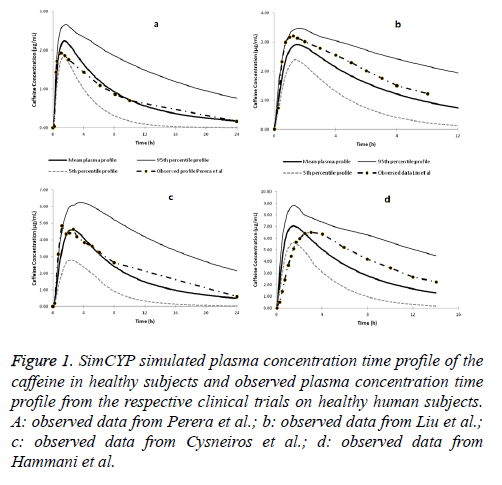
SimCYP simulated concentration time profile of caffeine in comparison with the observed data from the clinical trials is presented in Figures 1a-1d. All the four simulated clinical trials predictions matched with the observed data very well and were close to the predicted mean plasma concentration time profile. Moreover, all the mean of the concentration profiles from the observed clinical trials fell within the 95th and 5th percentile of the simulated concentration profiles of the respective trials. This confirms that the SimCYP PBPK model for the caffeine is good enough to be used to predict the caffeine disposition in healthy human subjects.
Figure 1: SimCYP simulated plasma concentration time profile of the caffeine in healthy subjects and observed plasma concentration time profile from the respective clinical trials on healthy human subjects. A: observed data from Perera et al.; b: observed data from Liu et al.; c: observed data from Cysneiros et al.; d: observed data from Hammani et al.
Phase 2 study results
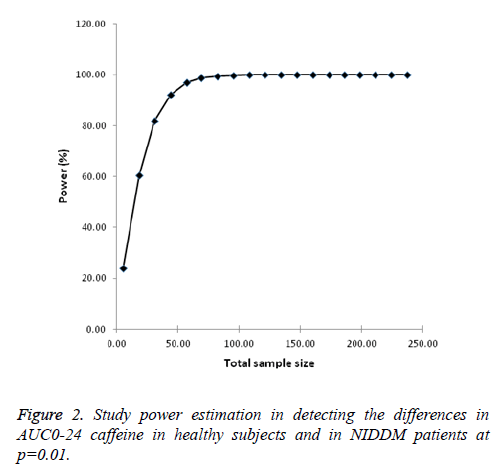
Power calculations: As expected power of the study to detect the changes in AUC0-24 improved with the sample size and the value converged at sample size of 121 with 100% power in each arm of the study. For phase 2 simulation studies, sample size used was set at 35 subjects each for both healthy and NIDDM patients which had a study power of more than 80% at significance of p=0.01 (Figure 2).
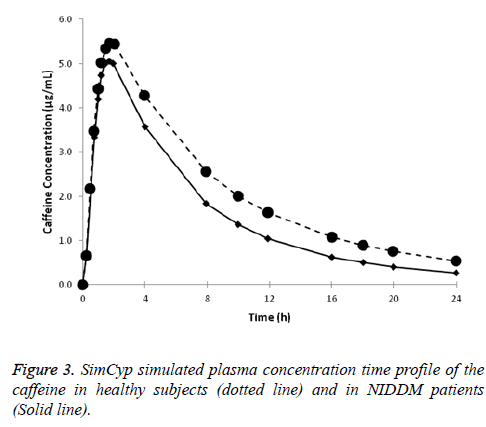
Caffeine disposition in diabetic patients: Plasma concentration-time profile for caffeine in healthy subjects in the diabetic patients obtained from the SimCYP simulations are presented in Figure 3. Clearance of caffeine in diabetic patients was 37% (p<0.01) more compared in healthy subjects as such the exposure of caffeine (AUC0-24 was 24% less, p<0.01) is less in comparison to that in healthy male subjects (Table 3).
| Parameter | Healthy subjects | Diabetic patients | Percentage difference in means | P value (Significance of difference) | ||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |||
| CL (L/h) | 5.43 | 4.05-5.74 | 7.45 | 5.60-7.89 | 37 | 0.007* |
| Vd (L/kg) | 0.49 | 0.43-0.51 | 0.49 | 0.43-0.51 | None | 1 |
| Cmax (μg/ml) | 5.55 | 4.88-5.86 | 5.14 | 4.50-5.43 | -18 | 0.09 |
| Tmax (h) | 1.91 | 1.79-1.99 | 1.77 | 1.66-1.85 | -7 | 0.03* |
| AUC (μg/ml.h) | 49.92 | 39.33-52.55 | 37.8 | 28.69-39.51 | -24 | 0.008* |
Table 3: SimCYP simulated pharmacokinetics of caffeine in healthy subjects and in diabetic patients.
Discussion
Results from this study indicate 37% rise in caffeine clearance in NIDDM patients in comparison to healthy, non-diabetic individuals of similar age. These results may also suggest similar increase in metabolism of CYP1A2 substrates in diabetic patients in comparison to healthy subjects as it is a common practice to drive clinical evidence on the CYP1A2 activity by studying the metabolism of caffeine as a probe drug. These results support the argument from in vitro and early animal studies that there is a variation in CYP1A2 activity in diabetic disease state. However Zysset et al. [24] failed to produce such effects perhaps due to the less sample size studies to pick the difference due to high variability in CYP1A2 enzyme abundance in humans. Power calculations from the current study also indicate that a sample size of about 31 subjects in each arm of the parallel design study comprising of group of healthy subjects and diabetic patients have a power of 80% to detect the difference in clearance between the two groups with 95% confidence in the results. None of the clinical studies conducted so far included number of subjects at this magnitude.
SimCYP simulation results points the lower exposure of CYP1A2 substrates in diabetic patients in comparison to healthy subjects. These effects potentially render a drug therapeutically ineffective in diabetic patients in case dose is based on standard pharmacokinetics in healthy subjects which is a usual clinical situation. This can lead to increase in the treatment cost especially with drugs with narrow safety margin. One of the possible explanations for lack of concluding clinical evidence for CYP1A2 activity in diabetic patients despite contradiction from clinical trials [24-26] is usually in clinical settings diminished activity or treatment failure is easily replaced by either increasing dose or by switching to another treatment alternatives. Whereas the situations of toxicities associated with increase in concentration are taken as a caution and steps to dose individualization are implicated if necessary. However therapeutic failure can cause extra treatment cost and failure of medical interventions when needed especially in emergency situations. Simulations with SimCYP by utilizing a validated PBPK model has an advantage to predict the clinical situations which are otherwise are overlooked due to cost and other reasons. This can also help to understand the underlying mechanisms and further may guide to design future studies [33].
Default SimCYP model for caffeine disposition was instrumental in matching the caffeine disposition from clinical trials on healthy Caucasian subjects. However with Saudi subjects SimCYP simulations for caffeine absorption after oral dose was faster than the observations from the clinical study by Hammani et al. [28]. This might be attributed to genetic differences between these pollutions. Caffeine disposition simulations from this study can be best being limited to Caucasian populations as the default data is from Caucasian population.
This simulation studies purposefully overlooked the physiological changes in diabetic patient which may affect the disposition of drugs as reported by Dostalek et al. and further illustrated by Li et al. [34,35]. Physiological changes reported viz., changes in gastric emptying, rise in blood flow to adipose tissues are expected to have minimal effect on caffeine disposition due to its rapid absorption and less lipid solubility. Moreover, purpose of this study was to investigate the changes in CYP1A2 activity in NIDDM patients and to extend the results to other CYP1A2 substrates. Matzke et al. [25] reported a 42% raise in CYP1A2 activity in NIDDM patients and also reported the tendency of increased renal clearance of 4- hydroxyantipyrine, CYP1A2 mediated metabolite of antipyrine, however both the results fail to reach statistical significance. Further to support the results of the current study Urry et al. recently reported a case-control study illustrating enhancement in caffeine clearance in NIDDM patients in comparison to healthy controls [36].
Conclusion
Default SimCYP model for caffeine is robust in predicting caffeine pharmacokinetics in healthy Caucasian subjects. Extension of the model to diabetic patients indicates decreased caffeine exposure in comparison with healthy subjects due to increased clearance (37%) for same dose. Results from this study suggest an increase in CYP1A2 activity in diabetic patients has potential to render CYP1A2 substrate ineffective. As such this study generates a caution for alteration in disposition of CYP1A2 substrates in diabetic patients in comparison to healthy subjects and guides for designing trials to detect the real effect in diabetic patients.
References
- Al Dawish MA, Robert AA, Braham R, Al Hayek AA, Al Saeed A, Ahmed RA. Diabetes mellitus in Saudi Arabia: a review of the recent literature. Curr Diabetes Rev 2015; 12: 359-368.
- Koloverou E, Panagiotakos DB, Pitsavos C, Chrysohoou C, Georgousopoulou EN, Pitaraki E. 10-year incidence of diabetes and associated risk factors in Greece: the ATTICA study (2002-2012). Rev Diabet Stud 2014; 11: 181-189.
- Liatis S, Dafoulas GE, Kani C, Politi A, Litsa P, Sfikakis PP. The prevalence and treatment patterns of diabetes in the Greek population based on real-world data from the nation-wide prescription database. Diabetes Res Clin Pract 2016; 118: 162-167.
- Turner M, Reid L, Munkonda M, Burger D. Os 02-03 effect of high glucose exposure on endothelial microparticle formation and composition. J Hypertens 2016; 34: 48.
- Sajja RK, Cucullo L. Altered glycaemia differentially modulates efflux transporter expression and activity in hCMEC/D3 cell line. Neurosci Lett 2015; 598: 59-65.
- Wang T, Shankar K, Ronis MJ, Mehendale HM. Mechanisms and outcomes of drug- and toxicant-induced liver toxicity in diabetes. Crit Rev Toxicol 2007; 37: 413-459.
- Haraya K, Kato M, Chiba K, Sugiyama Y. Prediction of inter-individual variability on the pharmacokinetics of CYP1A2 substrates in non-smoking healthy volunteers. Drug Metab Pharmacokinet 2016; 31: 276-284.
- Agundez JA, Garcia-Martin E, Alonso-Navarro H, Jimenez-Jimenez FJ. Anti-Parkinsons disease drugs and pharmacogenetic considerations. Expert Opin Drug Metab Toxicol 2013; 9: 859-874.
- Abdul MI, Jiang X, Williams KM, Day RO, Roufogalis BD, Liauw WS. Pharmacokinetic and pharmacodynamic interactions of echinacea and policosanol with warfarin in healthy subjects. Br J Clin Pharmacol 2010; 69: 508-515.
- Zhou SF, Yang LP, Zhou ZW, Liu YH, Chan E. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J 2009; 11: 481-494.
- Perera V, Gross AS, McLachlan AJ. Measurement of CYP1A2 activity: a focus on caffeine as a probe. Curr Drug Metab 2012; 13: 667-678.
- Syn NL, Yong WP, Lee SC, Goh BC. Genetic factors affecting drug disposition in Asian cancer patients. Expert Opin Drug Metab Toxicol 2015; 11: 1879-1892.
- Reeve E, Wiese MD, Mangoni AA. Alterations in drug disposition in older adults. Expert Opin Drug Metab Toxicol 2015; 11: 491-508.
- Fernandez Ortega A, Jolis Lopez L, Vinas Villaro G, Villanueva Vazquez R, Garcia Arias A, Gonzalez Farre X. Individualization of treatment strategies. Adv Ther 2011; 28: 19-38.
- Hartmanshenn C, Scherholz M, Androulakis IP. Physiologically-based pharmacokinetic models: approaches for enabling personalized medicine. J Pharmacokinet Pharmacodyn 2016; 43: 481-504.
- Jamei M, Marciniak S, Feng K, Barnett A, Tucker G, Rostami-Hodjegan A. The Simcyp population-based ADME simulator. Expert Opin Drug Metab Toxicol 2009; 5: 211-223.
- Gawronska-Szklarz B, Musial D, Drozdzik M, Paprota B. Metabolism of lidocaine by liver microsomes from streptozotocin-diabetic rats. Pol J Pharmacol 2003; 55: 251-254.
- Gawronska-Szklarz B, Musial HD, Loniewski I, Paprota B, Drozdzik M. Lidocaine metabolism in isolated perfused liver from streptozotocin-induced diabetic rats. J Pharm Pharmacol 2006; 58: 1073-1077.
- Lee DY, Lee MG, Shin HS, Lee I. Changes in omeprazole pharmacokinetics in rats with diabetes induced by alloxan or streptozotocin: faster clearance of omeprazole due to induction of hepatic CYP1A2 and 3A1. J Pharm Pharm Sci 2007; 10: 420-433.
- Oh SJ, Choi JM, Yun KU, Oh JM, Kwak HC, Oh JG. Hepatic expression of cytochrome P450 in type 2 diabetic Goto-Kakizaki rats. Chem Biol Interact 2012; 195: 173-179.
- Sindhu RK, Koo JR, Sindhu KK, Ehdaie A, Farmand F, Roberts CK. Differential regulation of hepatic cytochrome P450 monooxygenases in streptozotocin-induced diabetic rats. Free Radic Res 2006; 40: 921-928.
- Ueyama J, Wang D, Kondo T, Saito I, Takagi K, Takagi K. Toxicity of diazinon and its metabolites increases in diabetic rats. Toxicol Lett 2007; 170: 229-237.
- Sakuma T, Honma R, Maguchi S, Tamaki H, Nemoto N. Different expression of hepatic and renal cytochrome P450s between the streptozotocin-induced diabetic mouse and rat. Xenobiotica 2001; 31: 223-237.
- Zysset T, Wietholtz H. Pharmacokinetics of caffeine in patients with decompensated type I and type II diabetes mellitus. Eur J Clin Pharmacol 1991; 41: 449-452.
- Matzke GR, Frye RF, Early JJ, Straka RJ, Carson SW. Evaluation of the influence of diabetes mellitus on antipyrine metabolism and CYP1A2 and CYP2D6 activity. Pharmacotherapy 2000; 20: 182-190.
- Bechtel YC, Joanne C, Grandmottet M, Bechtel PR. The influence of insulin-dependent diabetes on the metabolism of caffeine and the expression of the debrisoquin oxidation phenotype. Clin Pharmacol Ther 1988; 44: 408-417.
- Lorusso PM, Boerner SA, Seymour L. An overview of the optimal planning, design, and conduct of phase I studies of new therapeutics. Clin Cancer Res 2010; 16: 1710-1718.
- Hammami MM, Al-Gaai EA, Alvi S, Hammami MB. Interaction between drug and placebo effects: a cross-over balanced placebo design trial. Trials 2010; 11: 110.
- Cysneiros RM, Farkas D, Harmatz JS, von Moltke LL, Greenblatt DJ. Pharmacokinetic and pharmacodynamic interactions between zolpidem and caffeine. Clin Pharmacol Ther 2007; 82: 54-62.
- Liu DJ, Kotler M, Sharples S. Pharmacokinetic and bioequivalence study evaluating a new paracetamol/caffeine formulation in healthy human volunteers. J Bioequival Bioavail 2011; 3: 251-257.
- Perera V, Gross AS, Forrest A, Landersdorfer CB, Xu H, Ait-Oudhia S. A pharmacometric approach to investigate the impact of methylxanthine abstinence and caffeine consumption on CYP1A2 activity. Drug Metab Dispos 2013; 41: 1957-1966.
- Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci 2006; 95: 1238-1257.
- Mohi Iqbal MA. Fatal France clinical trial and the lessons learned: Application of in silico approaches to investigate the disposition of B1A10-2474 and possible safety concerns. Tropical Journal of Pharmaceutical Research April 2017;16(4):911-7.
- Dostalek M, Akhlaghi F, Puzanovova M. Effect of diabetes mellitus on pharmacokinetic and pharmacodynamics properties of drugs. Clin Pharmacokinet 2012; 51: 481-499.
- Li J, Guo HF, Liu C, Zhong Z, Liu L, Liu XD. Prediction of drug disposition in diabetic patients by means of a physiologically based pharmacokinetic model. Clin Pharmacokinet 2015; 54: 179-193.
- Urry E, Jetter A, Landolt HP. Assessment of CYP1A2 enzyme activity in relation to type-2 diabetes and habitual caffeine intake. Nutr Metab (Lond) 2016; 13: 66.