ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 11
Luteolin enhances the antitumor activity of lapatinib in human breast cancer cells
Lingyan Zhang1,2, Fan Yang3, Li Huang4, Aixue Liu5 and Jiren Zhang1*
1Department of Oncology, Zhujiang Hospital, Southern Medical University, Guangzhou, PR China
2Medical Department, Chongqing Bishan District People’s Hospital, Chongqing, PR China
3Department of Basic Medicine, Xiangnan University, Chenzhou, PR China
4Department of Oncology, the First Affiliated Hospital of Gannan Medical University, Ganzhou, PR China
5Department of Oncology, the Second People’s Hospital of Shenzhen City, Shenzhen, PR China
- *Corresponding Author:
- Jiren Zhang
Department of Oncology
Zhujiang Hospital
Southern Medical University, PR China
Accepted date: March 27, 2017
Aims: The present study is to investigate the synergistic effect of lapatinib and luteolin in the inhibition of the growth and proliferation of breast cancer cells, as well as the underlying mechanisms.
Methods: Human breast cancer cell line BT474 was cultured in vitro. The cells were incubated with different concentrations of luteolin and/or lapatinib. MTS assay was used to test the proliferation of the cells. Flow cytometry was used to identify cell cycle. Quantitative real-time polymerase chain reaction was used to measure the expression of ERBB1 and ERBB2 mRNA. Western blotting was employed to determine the protein expression of ERBB1 and ERBB2. The phosphorylation of AKT, ERK1/2 and H2A.X, and the expression of Caspase 8 and PARP were also determined using Western blotting.
Results: Lapatinib, luteolin or the combination of both inhibited the proliferation of BT474 cells in a dose-dependent manner. Luteolin and lapatinib synergistically induced the apoptosis of BT474 cells. Combination of luteolin and lapatinib inhibited the expression of ERBB1 and ERBB2 mRNA and protein in BT474 cells. Combination of lapatinib and luteolin reduced the phosphorylation level of AKT and ERK1/2 in BT474 cells.
Conclusion: The present study demonstrates that synergistic use of luteolin with lapatinib inhibits the growth of breast cancer cells, possibly by inducing apoptosis via the deactivation of AKT and ERK signaling pathways.
Keywords
Luteolin, Lapatinib, Breast cancer, AKT, ERK
Introduction
Breast cancer is the most common malignant tumor in women, and its incidence all over the world is increasing year by year [1,2]. In China, the morbidity and mortality rates of breast cancer are also increasing [3]. Radical surgery is the most important method for the treatment of breast cancer. For patients with advanced tumors, molecular target therapy that is based on the key molecules in tumor cell growth and proliferation signaling pathway has become an important treatment method in addition to conventional therapies such as surgery, chemotherapy and radiotherapy [4,5]. Targeted therapeutic agents that have been applied to clinical practice include Epidermal Growth Factor Receptor (EGFR) inhibitors, monoclonal antibodies, and anti-tumor angiogenesis drugs [6-8].
Lapatinib is a kind of small-molecule tyrosine kinase inhibitor that binds with the ATP-binding sites of EGFR/Human epidermal growth factor receptor 2 (Her2) and inhibits their activation. In addition, lapatinib inhibits the activation of Mitogen-Activated Protein Kinases (MAPKs) and AKT downstream of EGFR/Her2, leading to suppressed cell proliferation and enhanced apoptosis [9]. In contrast to single tyrosine kinase inhibitor, lapatinib inhibits EGFR and Her2 at the same time, exerting synergistic effects on cell growth [10]. In the meantime, lapatinib prevents up-regulated expression of other members of Her family that is induced by treatment with single tyrosine kinase inhibitor.
Luteolin is a common type of natural flavonoid that is abundant in the leaves of celery, green pepper and dandelion. It possesses various biological activities such as anti-oxidation, anti-inflammation, anti-depression, anti-convulsion, antianxiety, and anti-anaphylaxis, and enhances immunity [11,12]. Luteolin has inhibitory effects on several types of malignant tumors. For example, luteolin inhibits the proliferation of lung adenocarcinoma A549 cells via PI3K-Akt-IkBα-Snail signaling pathway by down-regulating the expression of E-cadherin [13]. Luteolin suppresses the growth of colorectal cancer by regulating Wnt/β-catenin/GSK-3β signaling pathway and activating Nrf2 signaling pathway [14], and enhances the effect of tamoxifen on breast cancer by inhibiting the expression of cyclin E2 [15]. These studies suggest that luteolin can be a potential antitumor drug. However, the existence of synergistic effect of the combined use of lapatinib and luteolin has never been evaluated before. In the present study, we investigate whether the combined use of lapatinib and luteolin has synergistic effect on human breast cancer cells, as well as the underlying mechanism.
Materials and Methods
Cells
Human breast cancer cell line BT474 (American Type Culture Collection, Manassas, VA, USA) was cultured in RPMI-1640 medium containing 2 mmol/L glutamine, 10% fetal bovine serum, 100 μg/ml streptomycin and 100 U/ml penicillin G at 37°C and 5% CO2. When reaching confluency, the cells were trypsinized and centrifuged at 1,000 rpm for 5 min. Then, the cells were seeded into 6-well or 24-well plates according to different experimental purposes. The cell density was adjusted to 105/ml. During the whole culture procedure, mycoplasma examination was performed to exclude contamination.
MTS cell proliferation assay
BT474 cells were prepared into single cell suspension in RPMI-1640 medium containing 10% fetal bovine serum. About 1,000 cells (100 μl) were inoculated into each well of 96-well plates, and cultured for 72 h. After incubation, MTS solution (20 μl) was added into each well for incubation for 2-4 h. Then, absorbance of each well was measured at 490 nm. Cell growth curves were plotted using absorbance as Y axis and time as X axis. The inhibition rates of each group of cells and IC50 values of different concentrations of drugs were calculated. Using isobologram analysis, Combination Index (CI) of drugs was calculated [16]. If CI>1, the two drugs had antagonistic effect; if CI=1, the two drugs had additive effect; if CI<1, the two drugs had synergistic effect.
Flow cytometry
A total of 1 × 105 cells were collected and centrifuged at 1,000 rpm for 5 min. After discarding supernatant, the cells were washed with Phosphate-Buffered Saline (PBS) before addition of ice cold 70% ethanol for fixation. After incubation at 4°C overnight, the cells were centrifuged at 1,000 rpm for 5 min and washed with PBS once. Then, the cells were stained using 1 ml propidium iodide and incubated at 4°C in dark for 30 min before flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA). The data were analyzed using Modifit LT software (BD Biosciences, Franklin Lakes, NJ, USA).
Western blotting
The cells were collected and washed with sterile PBS. Proteins were extracted from nucleus and cytoplasm using protein extraction kit (Pierce, Thermo Fisher Scientific, Waltham, MA, USA). After determination of protein concentration, the samples were mixed with equal volume of 2X sodium dodecyl sulfate loading buffer before boiling for 5 min. Then, 20 μl protein was subjected to sodium dodecyl sulfatepolyacrylamide gel electrophoresis. The resolved proteins were transferred to polyvinylidene difluoride membranes on ice (300 mA, 2 h) and blocked with 5% skimmed milk at room temperature for 2 h. Then, the membranes were incubated with mouse anti-human phosphorylated and total Akt (1:1,000, #12694; 1:2,000, #2920) and ERK1/2 (1:1,000, #9107; 1:2,000, #4696) monoclonal bodies, mouse anti-human PARP monoclonal body (1:2,000, #9546), rabbit anti-human γ-H2A.X monoclonal primary antibodies (1:2,000, #5438, Cell Signaling Technology, Danvers, MA, USA), rat anti-human EGFR monoclonal (1:500, sc-71035) and mouse anti-human Her2 monoclonal antibodies (1:2,000; sc-33684, Santa Cruz Biotechnology, Dallas, TX, USA) and mouse anti-human β- actin monoclonal primary antibody (1:2,000; #A5316, Sigma- Aldrich, St. Louis, MO, USA) at 4°C overnight. After extensive washing with PBS with Tween 20 for 3 times of 15 min, the membranes were incubated with polyclonal rabbit anti-mouse (1:5,000, ab6728), rabbit anti-rat (1:4,000, ab6734) or goat anti-rabbit (1:5,000, ab6721) horseradish peroxidaseconjugated secondary antibodies (Abcam, Cambridge, UK) for 1 h at room temperature before washing with PBS with Tween 20 for 3 times of 15 min. Then, the membrane was developed with enhanced chemiluminescence detection kit (Millipore, Billerica, MA, USA) for imaging (VL Chemi-Smart 3000; Viogene Biotek, Sunnyvale, CA, USA). The relative content of target protein was measured against β-actin or the endogenous level of total kinase protein.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Cells were trypsinized and mixed with 1 ml Trizol for lysis. Then, total RNA was extracted using phenol chloroform method. The purity of RNA was determined by A260/A280 using ultraviolet spectrophotometry (Nanodrop ND2000, Thermo Scientific, Waltham, MA, USA). Then, cDNA was obtained by reverse transcription using Reverse Transcription System (Takara, Dalian, China) from 1 μg RNA and stored at -20°C. After addition of 10 mmol/L forward and reverse primers and SYBR (Invitrogen, Carlsbad, CA, USA), qRTPCR was carried out on ABI 7500 instrument (Thermo Fisher Scientific, Waltham, MA, USA). The primer sequences were: ERBB1, 5’-AGGACCAAGCAACATGGTCA-3’ (forward) and 5’-CCTTGCAGCTGTTTTCACCT-3’ (reverse); ERBB2, 5’-CACAAAAGTGAGTGTGCACCGGC-3’ (forward) and 5’-CAGGCTGGCATTGGTGGGCA-3’ (reverse); GAPDH, 5’- ATGGGGAAGGTGAAGGTCG-3’ (forward) and 5’- GGGTCATTGATGGCAACAATATC-3’ (reverse) [17]. The 2-ΔΔCt method was used to calculate the relative expression of target mRNA against GADPH. Each sample was tested in triplicate.
Statistical analysis
All results were analysed using SPSS 17.0 statistical software (IBM, Armonk, NY, USA). Measurement data were expressed as means ± SEM. Comparison among multiple groups was performed using analysis of variance. Differences with P<0.05 were considered statistically significant.
Results
Lapatinib, luteolin or the combination of both inhibits the proliferation of BT474 cells in a dose-dependent manner
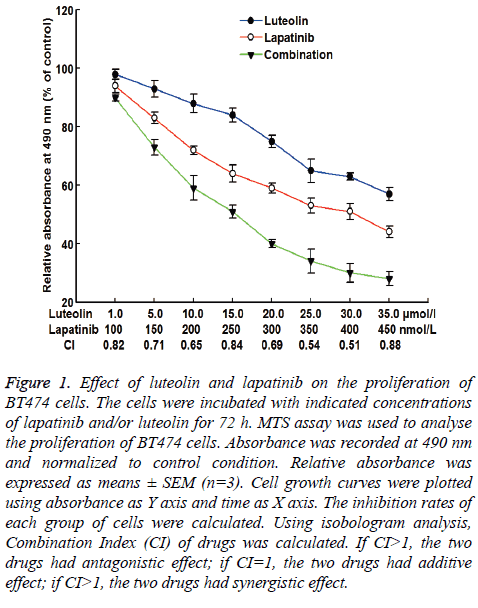
To test the effect of lapatinib, luteolin or the combination of both drugs on the proliferation of BT474 cells, MTS assay was carried out. The data showed that the relative absorbance at 490 nm was decreased as the doses of individual or combined drugs were increasing. The decrease had a dose-dependent manner. When the dose of luteolin was 15 μmol/L and the dose of lapatinib was 250 nmol/L, the inhibition rate of BT474 cell growth was 52%, with a CI of 0.84 (Figure 1). Therefore, 15 μmol/L and 250 nmol/L were chosen as study doses for luteolin and lapatinib, respectively, in the following tests. These results suggest that lapatinib, luteolin or the combination of both inhibits the proliferation of BT474 cells in a dosedependent manner.
Figure 1: Effect of luteolin and lapatinib on the proliferation of BT474 cells. The cells were incubated with indicated concentrations of lapatinib and/or luteolin for 72 h. MTS assay was used to analyse the proliferation of BT474 cells. Absorbance was recorded at 490 nm and normalized to control condition. Relative absorbance was expressed as means ± SEM (n=3). Cell growth curves were plotted using absorbance as Y axis and time as X axis. The inhibition rates of each group of cells were calculated. Using isobologram analysis, Combination Index (CI) of drugs was calculated. If CI>1, the two drugs had antagonistic effect; if CI=1, the two drugs had additive effect; if CI>1, the two drugs had synergistic effect.
Luteolin and lapatinib synergistically induce the apoptosis of BT474 cells
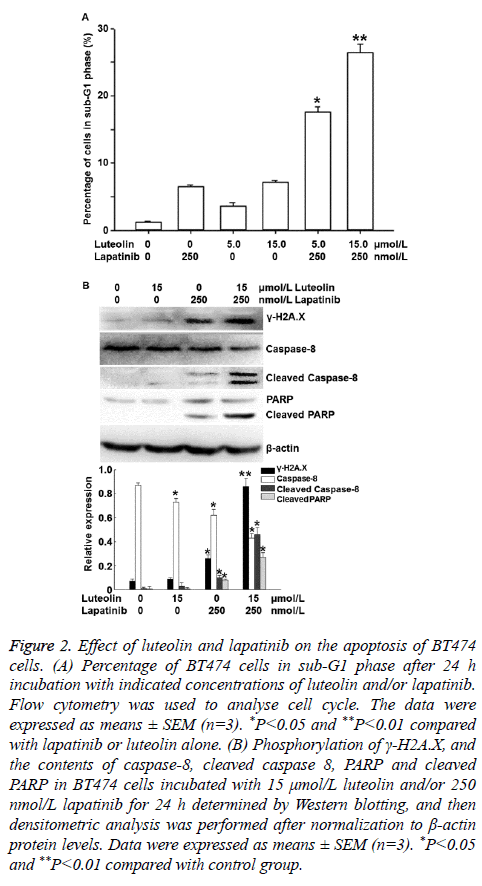
To investigate the effects of lapatinib and luteolin on the cell cycle and apoptosis of BT474 cells, flow cytometry and Western blotting were performed. Flow cytometry showed that incubation with 5 or 15 nmol/L luteolin alone increased the percentages of cells in sub-G1 phase, and combined use of 5 or 15 nmol/L luteolin with 250 nmol/L lapatinib significantly increased the percentages of cells in sub-G1 phase to 17.6% and 26.4%, respectively (P<0.05) (Figure 2A). Western blotting showed that incubation with 15 μmol/L luteolin alone failed to significantly alter the phosphorylation of γ-H2A.X, or the contents of caspase 8 or PARP. Similarly, incubation with 250 μmol/L lapatinib alone only slightly affected the phosphorylation of γ-H2A.X, as well as the contents of caspase 8 and PARP. Of note, combined use of 15 μmol/L luteolin and 250 μmol/L lapatinib significantly enhanced the phosphorylation level of γ-H2A.X, and the contents of cleaved Caspase 8 (active format of caspase 8) and cleaved PARP (death substrate) (P<0.05) (Figure 2B). These results indicate that luteolin and lapatinib synergistically induce the apoptosis of BT474 cells.
Figure 2: Effect of luteolin and lapatinib on the apoptosis of BT474 cells. (A) Percentage of BT474 cells in sub-G1 phase after 24 h incubation with indicated concentrations of luteolin and/or lapatinib. Flow cytometry was used to analyse cell cycle. The data were expressed as means ± SEM (n=3). *P<0.05 and **P<0.01 compared with lapatinib or luteolin alone. (B) Phosphorylation of γ-H2A.X, and the contents of caspase-8, cleaved caspase 8, PARP and cleaved PARP in BT474 cells incubated with 15 μmol/L luteolin and/or 250 nmol/L lapatinib for 24 h determined by Western blotting, and then densitometric analysis was performed after normalization to β-actin protein levels. Data were expressed as means ± SEM (n=3). *P<0.05 and **P<0.01 compared with control group.
Combination of luteolin and lapatinib inhibits the expression of ERBB1 and ERBB2 mRNA and protein in BT474 cells
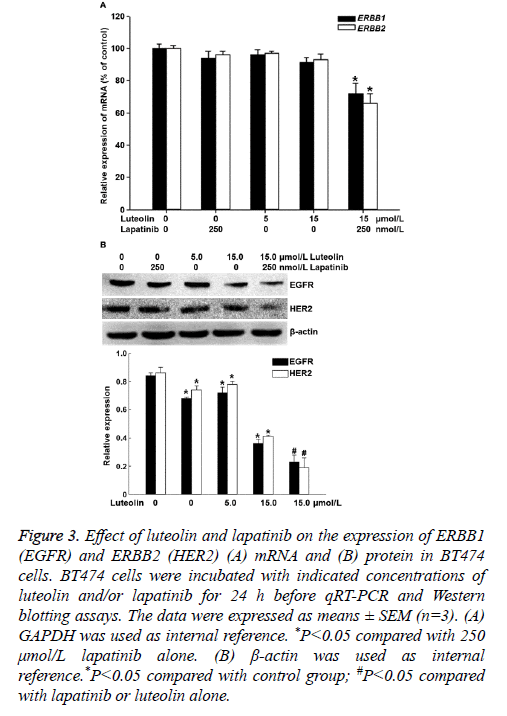
To measure the expression of ERBB1 (EGFR) and ERBB2 (HER2) mRNA and protein, qRT-PCR and Western blotting were employed. The data showed that treatment with 5 μmol/L luteolin, 15 μmol/L luteolin or 250 μmol/L lapatinib alone for 24 h failed to significantly alter the expression of ERBB1 or ERBB2 mRNA. By contrast, combined use of 15 μmol/L luteolin and 250 μmol/L lapatinib significantly decreased the expression of both ERBB1 and ERBB2 mRNA (P<0.05) (Figure 3A). In addition, treatment with 5 μmol/L or 15 μmol/L luteolin alone for 24 h slightly decreased the protein expression of EGFR and HER2. Of note, combined use of 15 μmol/L luteolin and 250 μmol/L lapatinib further decreased the expression of both EGFR and HER2 proteins (P<0.05) (Figure 3B). The results suggest that the combination of luteolin and lapatinib inhibits the expression of ERBB1 and ERBB2 mRNA and protein in BT474 cells.
Figure 3: Effect of luteolin and lapatinib on the expression of ERBB1 (EGFR) and ERBB2 (HER2) (A) mRNA and (B) protein in BT474 cells. BT474 cells were incubated with indicated concentrations of luteolin and/or lapatinib for 24 h before qRT-PCR and Western blotting assays. The data were expressed as means ± SEM (n=3). (A) GAPDH was used as internal reference. *P<0.05 compared with 250 μmol/L lapatinib alone. (B) β-actin was used as internal reference.*P<0.05 compared with control group; #P<0.05 compared with lapatinib or luteolin alone.
Combination of lapatinib and luteolin reduces the phosphorylation levels of AKT and ERK1/2 in BT474 cells
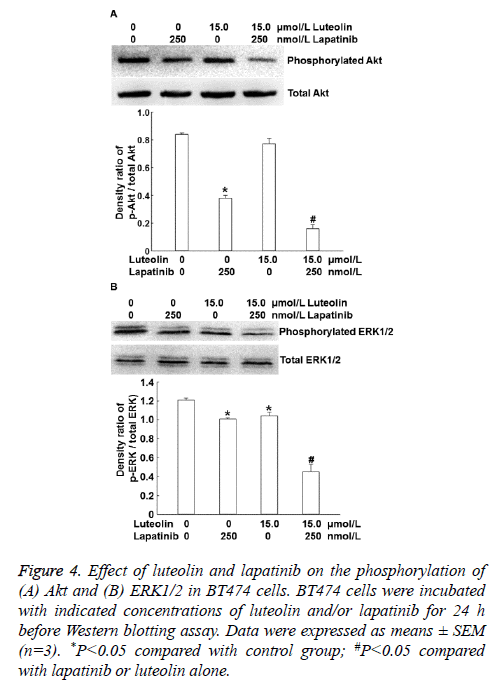
To identify the mechanism by which the combination of luteolin and lapatinib on the proliferation of BT474 cells, we examined the PI3K/Akt and ERK1/2 signaling pathways that are closely related with HER. Western blotting showed that 15 μmol/L luteolin alone slightly affected the level of intracellular phosphorylated AKT and 250 nmol/L lapatinib alone reduced the level of intracellular phosphorylated AKT compared with that in the absence of drug treatment. By contrast, combined use of 250 nmol/L lapatinib and 15 μmol/L luteolin dramatically decreased the level of intracellular phosphorylated AKT (P<0.05) (Figure 4A). Moreover, treatment with 15 μmol/L luteolin alone slightly affected the phosphorylation level of ERK1/2, and 250 nmol/L lapatinib alone reduced the phosphorylation level of ERK1/2. Of note, combined use of 250 nmol/L lapatinib and 15 μmol/L luteolin further decreased the phosphorylation level of ERK1/2 (P<0.05) (Figure 4B). The results indicate that combination of lapatinib and luteolin may inhibit the proliferation of BT474 cells by affecting the phosphorylation levels of PI3K/Akt and ERK1/2.
Figure 4: Effect of luteolin and lapatinib on the phosphorylation of (A) Akt and (B) ERK1/2 in BT474 cells. BT474 cells were incubated with indicated concentrations of luteolin and/or lapatinib for 24 h before Western blotting assay. Data were expressed as means ± SEM (n=3). *P<0.05 compared with control group; #P<0.05 compared with lapatinib or luteolin alone.
Discussion
Due to the continuous development of new drugs, the survival rate of breast cancer patients has been greatly improved. However, these therapeutic agents often target at single sites. Signal pathways in the body are complexed, so the effect of single administration of targeted drugs is usually limited [18,19]. Combined use of drugs that target multiple pathways in the process of tumor cell growth and invasion may have more effective and lasting inhibitory effects on tumor cells [20]. As a tyrosine kinase inhibitor, lapatinib inhibits the activity of intracellular EGFR and Her2 by inhibiting their ATP sites [21]. It is reported that cetuximab enhances the sensitivity of colon cancer patients with irinotecan resistance [22]. In addition, cetuximab and mapatumumab improve the clinical prognosis of wild-type KRAS patients when used in conjugation with oxaliplatin and irinotecan, respectively [23]. These studies suggest that EGFR may be a key reason for the resistance of various patients to a variety of drugs [24,25]. Our results in the present study show that luteolin and lapatinib synergistically inhibit the growth of breast cancer cells. In addition, treatment with luteolin or lapatinib alone fails to induce apoptosis, whereas combined use of both drugs arrests cell cycle and induces apoptosis. Apoptosis is demonstrated by the phosphorylation of γ-H2AX and the activation of Caspase family proteins [26]. Phosphorylation of γ-H2AX is the symbol of early apoptosis, while Caspase family proteins abolish the enzymatic activity of DNA repair enzyme PARP [27]. There are two anti-apoptotic signaling pathways in cells, including EGFR-MAPK signaling pathway and PI3K-AKT signaling pathway [28,29]. In healthy cells, the activities of the two signaling pathways are low, and they only maintain normal growth and proliferation of cells. Our results show that the synergistic inhibitory effect of luteolin on breast cancer cell growth is related with the phosphorylation of PI3K and ERK. Treatment with luteolin reduces cell activities and increases their sensitivity to lapatinib. EGFR, Raf and AKT are substrates for HSP90, and binding with EGFR, Raf or AKT maintains the stable status of HSP90 [30]. Some histone deacetylase inhibitors can induce the deacetylation of HSP90, and the dissociation of HSP90 with its substrates releases EGFR, Raf or AKT, which can be degraded via proteasome pathway, inhibiting the activation of anti-apoptotic signaling pathway and promoting the apoptosis of lung cancer cells [31]. In ovarian cancer cells that are resistant to cisplatin, luteolin further enhances their sensitivity to chemotherapeutic drugs [32]. However, whether luteolin synergistically enhances the anti-tumor effect of lapatinib via HSP90 still needs further studies.
Complexed interactions with EGFR and other members of ERBB receptor family may affect the sensitivity of EGFRtargeted drugs on tumor cells. Therefore, we have investigated the effect of the combination of luteolin and lapatinib on the expression of EGFR and Her2 mRNA. The result shows that combined use of luteolin and lapatinib reduces the expression of EGFR and Her2 mRNA and proteins. It is reported that inhibition of HSP90 and treatment with lapatinib synergistically suppress the growth of colon cancer cells [17]. This suggests that unfolded protein response may play important roles in EGFR/Her2 signaling pathway, and EGFR and Her2 may both be the substrates of HSP90 [31]. In addition, luteolin is shown to bind with HSP90, and induces tumor cell apoptosis by inhibiting the sustained activation of STAT3 [33]. In conclusion, the present study demonstrates that combined use of lapatinib and luteolin inhibits the growth of BT474 cells, and the two drugs have synergistic effects. Treatment with lapatinib and luteolin down-regulates the expression of EGFR and Her2 in BT474 cells, inhibits the activation of PI3K and ERK signaling pathways, and promotes apoptosis. However, the mechanism of the synergistic effect of lapatinib and luteolin is complexed and still needs further studies in the future.
Acknowledgements
This work was supported by the Medical Scientific Research Project of Health and Family Planning Commission of Chongqing City (No. 20142203).
Disclosures
All authors declare no financial competing interests. All authors declare no non-financial competing interests.
References
- Kohler LN, Garcia DO, Harris RB, Oren E, Roe DJ, Jacobs ET. Adherence to diet and physical activity cancer prevention guidelines and cancer outcomes: a systematic review. Cancer Epidemiol Biomarkers Prev 2016; 25: 1018-1028.
- Nigenda G, Gonzalez-Robledo MC, Gonzalez-Robledo LM, Bejarano-Arias RM. Breast cancer policy in Latin America: account of achievements and challenges in five countries. Global Health 2016; 12: 39.
- Lock M, Kaufert P. Menopause, local biologies, and cultures of aging. Am J Hum Biol 2001; 13: 494-504.
- Lee DW, Lee KH, Kim JW, Keam B. Molecular targeted therapies for the treatment of leptomeningeal carcinomatosis: current evidence and future directions. Int J Mol Sci 2016; 17.
- Zanardi E, Bregni G, de Braud F, Di Cosimo S. Better together: targeted combination therapies in breast cancer. Semin Oncol 2015; 42: 887-895.
- Davis CC, Zelnak A, Eley JW, Goldstein DA, Switchenko JM. Clinical utility of routine cardiac monitoring in breast cancer patients receiving trastuzumab. Ann Pharmacother 2016; 50: 712-717.
- Sawaki M, Ito Y, Tada K, Mizunuma N, Takahashi S. Efficacy and safety of trastuzumab as a single agent in heavily pretreated patients with HER-2/neu-overexpressing metastatic breast cancer. Tumori 2004; 90: 40-43.
- Thallinger C, Lang I, Kuhar CG, Bartsch R. Phase II study on the efficacy and safety of Lapatinib administered beyond disease progression and combined with vinorelbine in HER-2/neu- positive advanced breast cancer: results of the CECOG LaVie trial. BMC Cancer 2016; 16: 121.
- Liu CY, Hu MH, Hsu CJ. Lapatinib inhibits CIP2A/PP2A/p-Akt signaling and induces apoptosis in triple negative breast cancer cells. Oncotarget 2016; 7: 9135-9149.
- Spector NL, Robertson FC, Bacus S, Blackwell K, Smith DA. Lapatinib plasma and tumor concentrations and effects on Her receptor phosphorylation in tumor. PLoS One 2015; 10: 0142845.
- Nabavi SF, Braidy N, Gortzi O, Sobarzo-Sanchez E, Daglia M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res Bull 2015; 119: 1-11.
- Liu CW, Lin HW, Yang DJ. Luteolin inhibits viral-induced inflammatory response in RAW264.7 cells via suppression of STAT1/3 dependent NF-κB and activation of HO-1. Free Radic Biol Med 2016; 95: 180-189.
- Chen KC, Chen CY, Lin CR. Luteolin attenuates TGF-β1-induced epithelial-mesenchymal transition of lung cancer cells by interfering in the PI3K/Akt-NF-κB-Snail pathway. Life Sci 2013; 93: 924-933.
- Ashokkumar P, Sudhandiran G. Luteolin inhibits cell proliferation during Azoxymethane-induced experimental colon carcinogenesis via Wnt/β-catenin pathway. Invest New Drugs 2011; 29: 273-284.
- Tu SH, Ho CT, Liu MF. Luteolin sensitises drug-resistant human breast cancer cells to tamoxifen via the inhibition of cyclin E2 expression. Food Chem 2013; 141: 1553-1561.
- Kim HG, Shrestha B, Lim SY. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol 2006; 545: 192-199.
- Labonte MJ, Wilson PM, Fazzone W. The dual EGFR/HER2 inhibitor lapatinib synergistically enhances the antitumor activity of the histone deacetylase inhibitor panobinostat in colorectal cancer models. Cancer Res 2011; 71: 3635-3648.
- Ling J, Kumar R. Crosstalk between NFkB and glucocorticoid signaling: a potential target of breast cancer therapy. Cancer Lett 2012; 322: 119-126.
- Riccio G, Coppola C, Piscopo G, Capasso I, Maurea C. Trastuzumab and target-therapy side effects: Is still valid to differentiate anthracycline Type I from Type II cardiomyopathies? Hum Vaccin Immunother 2016; 12: 1124-1131.
- Lee SY, Oh SC. Changing strategies for target therapy in gastric cancer. World J Gastroenterol 2016; 22: 1179-1189.
- Gilmer TM, Cable L, Alligood K, Rusnak D, Spehar G. Impact of common epidermal growth factor receptor and HER2 variants on receptor activity and inhibition by lapatinib. Cancer Res 2008; 68: 571-579.
- Janjigian YY, Ku GY, Campbell JC. Phase II trial of cetuximab plus cisplatin and irinotecan in patients with cisplatin and irinotecan-refractory metastatic esophagogastric cancer. Am J Clin Oncol 2014; 37: 126-130.
- Berlin J, Posey J, Tchekmedyian S, Hu E, Chan D. Panitumumab with irinotecan/leucovorin/5-fluorouracil for first-line treatment of metastatic colorectal cancer. Clin Colorectal Cancer 2007; 6: 427-432.
- Chen SJ, Luan J, Zhang HS, Ruan CP, Xu XY. EGFR-mediated G1/S transition contributes to the multidrug resistance in breast cancer cells. Mol Biol Rep 2012; 39: 5465-5471.
- Jin Y, Zhang W, Wang H. EGFR/HER2 inhibitors effectively reduce the malignant potential of MDR breast cancer evoked by P-gp substrates in vitro and in vivo. Oncol Rep 2016; 35: 771-778.
- Chiu SJ, Chao JI, Lee YJ, Hsu TS. Regulation of gamma-H2AX and securin contribute to apoptosis by oxaliplatin via a p38 mitogen-activated protein kinase-dependent pathway in human colorectal cancer cells. Toxicol Lett 2008; 179: 63-70.
- Simbulan-Rosenthal CM, Rosenthal DS, Iyer S, Boulares H, Smulson ME. Involvement of PARP and poly(ADP-ribosyl)ation in the early stages of apoptosis and DNA replication. Mol Cell Biochem 1999; 193: 137-148.
- Sarkar S, Mazumdar A, Dash R, Sarkar D, Fisher PB, Mandal M. ZD6474, a dual tyrosine kinase inhibitor of EGFR and VEGFR-2, inhibits MAPK/ERK and AKT/PI3-K and induces apoptosis in breast cancer cells. Cancer Biol Ther 2010; 9: 592-603.
- Zhou Y, Li S, Hu YP. Blockade of EGFR and ErbB2 by the novel dual EGFR and ErbB2 tyrosine kinase inhibitor GW572016 sensitizes human colon carcinoma GEO cells to apoptosis. Cancer Res 2006; 66: 404-411.
- Musso L, Dallavalle S, Merlini L, Bava A, Nasini G. Natural and semisynthetic azaphilones as a new scaffold for Hsp90 inhibitors. Bioorg Med Chem 2010; 18: 6031-6043.
- Shimamura T, Lowell AM, Engelman JA, Shapiro GI. Epidermal growth factor receptors harboring kinase domain mutations associate with the heat shock protein 90 chaperone and are destabilized following exposure to geldanamycins. Cancer Res 2005; 65: 6401-6408.
- Dia VP, Pangloli P. Epithelial-to-mesenchymal transition in paclitaxel-resistant ovarian cancer cells is downregulated by luteolin. J Cell Physiol 2017; 232: 391-401.
- Fu J, Chen D, Zhao B. Luteolin induces carcinoma cell apoptosis through binding Hsp90 to suppress constitutive activation of STAT3. PLoS One 2012; 7: 49194.