ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 1
Iontophoretic delivery of lignocaine in healthy volunteers: effect of concentration of epinephrine on local anesthesia
1Department of Anesthesiology, The second people’s hospital of Jiaozuo, PR China
2Department of Anesthesiology, The Chinese people’s liberation army air force general hospital, PR China
- *Corresponding Author:
- Feng Liu
Department of Anesthesiology
The second people’s hospital of Jiaozuo
PR China
Accepted date: May 16, 2016
The current clinical study focuses on the effect of concentration of epinephrine on the anesthetic activity of Lignocaine delivered by iontophoresis. The combination of drugs is supposed to lower the intensity of pain with lowering in various physical stimuli. The patches for drug reservoir (Lignocaine and Epinephrine) and electrolyte were formulated by casting solvent and evaporation method. The controlled, randomized trial was conducted on 30 subjects (15 male and 15 females) with mean age 52 years and mean height 158 cm. The total population was divided into 3 random groups and treated with varying concentration of epinephrine as AT1 (2% Lignocaine + 1:80000), AT2 (2% Lignocaine + 1:160000), AC (2% Lignocaine). The Lignocaine epinephrine patches were formulated successfully by casting solvent and evaporation method. AT2 group gave significantly less pain intensity than AT1 and AC group. The cool and warm sensation observed for AT2 group was significantly lowered than AT1 and AC group. All the formulation showed no significant skin reaction. High epinephrine concentration combined with Lignocaine offers significant advantage for pain relief. As concentration of Epinephrine increases the pain relieving effect also increases.
Keywords
Anesthesia, Iontophoresis, Lignocaine, Epinephrine, Pain, Transdermal patch.
Introduction
Topical delivery of drugs via skin has always been a great challenge for investigators due to structure of skin as outermost layer stratum corneum exhibit barrier properties for many drugs [1] still the delivery system is a lot favourite due to significant clinical benefits such as predetermined release rate, achievement of steady state plasma concentration, pain free self-application, bypassing first pass metabolism etc. [2]. But the system is found most efficacious for drug or drug with penetration enhancer for highly potent lipophilic drug molecules with low molecular weight. So, various strategies have been implemented for enhancement of drug permeability through various skin barriers including stratum corneum (SC). Among the available various approaches Iontophoresis (IP) is the most acceptable physical method exerting its electrical effects on skin, SC and beneath layers of skin [3].
A medicated adhesive patch or transdermal patch is device meant to be placed over the skin surface and intended to deliver medicament through skin and into bloodstream providing advantage of controlled release of drug for prolong period of time, with reduced dosing and improved therapeutic efficacy and maintenance of constant plasma drug levels. The patch can be either a porous membrane or reservoir type. These patches are now proving to be alternative to for existing therapeutic systems [4].
Iontophoresis (IP) is a physical method under topical delivery which involves transfer of charged drug molecules under the influence of electric current in to biological barriers and various tissues. This current leads to increase in movement of ion across biological membrane using externally applied potential difference. IP is usually operated at very low voltage of 0.1-5 V and at constant current of 0.5 mA/cm2 over prolonged period of time. The electric current induced in skin is responsible for the transportation of drug molecules or its salt from through skin barriers. The amount and distribution of delivered drug is dependent on ion charge, drug concentration, current intensity, contact surface area to electrode etc [5]. When the biological membrane involved in process is skin the method is known as transdermal iontophoresis [6]. Transdermal iontophoresis drives the drug molecule carrying similar charge as active electrode by repulsion across skin. The amount of the drug delivered across the skin is directly proportional to the applied electric charge, applied time and duration of application [7]. Iontophoretic delivery of Lignocaine appears to be a promising approach for rapid onset of anaesthesia [8].
After administration of Local anaesthetic (LA) the multiple actions on muscles and nerves takes place that leads to net increase or decrease circulation which may removes the drugs from site of action. Furthermore, the blood flow changes can be reversed over time thereby naturally decreasing local distribution of body tissues, lowering concentration of plasma LA [9]. The vasomotor effects of the Lignocaine are concentration dependent.
The Epinephrine, and sympathomimetic amine interacting with adrenergic receptors, shows unequivocal relationship between concentration and pain after surgery. When administerd exogenously it shows induction in pain. The agent added to LA in order to prolong the action via vasoconstrictor effect resulting in reduction of tissue perfusion and oxygen availability [10].
So, anaesthesiologist prefer the combination of epinephrine to Lignocaine during peripheral nerve block procedures as system offers advantages of reduction of LA plasma concentration nullifying the chances of systemic toxicity plus enhancing quality and duration of anaesthesia [11]. The concept has been accepted that epinephrine exerts this effect via its vasoconstrictor action through adrenergic receptors on neural vasculature. The effects like smooth muscle contraction, decreased blood flow resulting in reduced Lignocaine clearance. The increase LA block by epinephrine when combine with Lignocaine (by enhancement of submaximal LA doses) could be the result of pharmacokinetic factors that increase intraneural LA concentration or pharmacodynamics actions on nerve membrane [12,13].
Materials
Lignocaine and Epinephrine was generously gifted by Shouguang Fukang Pharmacy Factory (Shandong, China). Polyvinyl alcohol (PVA), Dibutyl phthalate (DBT), Hydroxy propyl methyl cellulose (HPMC) were purchased from sigma Aldrich; USA. All other chemical agents and materials were of analytical grade and used as received. LidoSite™ Controller system purchased from Amsterdam pharmacy (USA).
Methods
Preparation of patch enclosing local anaesthetic and epinephrine
The Transdermal patch of LA and epinephrine was prepared by using solvent casting and evaporation method in which PVA is used as polymer. The 5% solution of PVA was dissolved in purified water. Thus prepared solution was poured in glass petri dish (whose diameter was previously measured) to form the backing membrane of the patch. The dish was then dried in hot air oven at 50ºC. The drug solutions were prepared by dissolving the predetermined amount of drugs in methanolic solution (solution A). While preparing the drug solutions the concentration of Lignocaine was made fixed while Epinephrine concentration kept A1 (1:80000), A2 (1: 160000). Another patch containing only Lignocaine as drug was also prepared named as AC (i.e. control formulation). The polymeric solution was prepared by dissolving HPMC in distilled water with addition of dibutyl phthalate (solution B). The solution A was added to solution B drop wise using magnetic stirrer and poured on the petri dish previously containing backing membrane. The plate was dried for 4-5 hr at 55ºC. When patch was dried it was removed from plate to cut in to small pieces. Thus prepared patch was evaluated for various parameters [14].
The patches were prepared using 3 different concentrations of Epinephrine and labeled as: AT1 (Lignocaine 2%+1:80 000), AT2 (Lignocaine 2%+1:160 000), AC (Lignocaine 2% only).
Preparation of patch enclosing electrolyte solution
The electrolyte reservoir was prepared by same method as described above which contains glycerin, sodium chloride, preservatives (2-phenoxyethanol, methyl-,ethyl-, propyl-,butyl-,and isobutyl-p-hydroxybenzoate) and monobasic sodium phosphate.
Evaluation of patches
Preliminary physical evaluation: The physical evaluation was carried out for all the patches. The patches were inspected for visually for clarity, color and smoothness. The thickness of the patches was determined by using screw gauze at 5 different locations and the average was calculated. The weight variation of the patches was determined by measuring the weight of the patches from each batch and average weight of the patch was determined. The strength of the patch was determined by using the folding endurance test which was performed by repeatedly folding the patch at the same place until it broke. The folding endurance value was considered the number of times the patch could be folded at the same place without breaking or cracking. Mechanical properties and strength of the patches were determined by measuring their tensile strength. Tensile strength of the patch was determined with Universal strength testing apparatus.
Content uniformity: To assure the complete distribution of drug throughout the patch the test was performed on 10 patches that are previously soaked in 25 ml methanolic solution. This methanolic solution was filtered through 0.3 micron filter to remove the undissolved residue of the polymer and content uniformity was determined using HPLC. The HPLC system consisted UV-visible detector (SPD-10A). A filtrate of 15 μL was injected into HPLC system containing C18 reverse phase column using mobile phase of acetonitrile and phosphate buffer pH 7.4 (95:5 V/V). The flow rate was 1.0 ml/min with detection at 220 nm.
Percentage moisture absorption: The 5 patches were weighed (W1, initial weight) accurately and placed in vaccume desiccator for 5 days. After completion of time period the patches were taken out and reweighed (W2, weight after desiccation). The percent moisture uptake was calculated from the difference between (W1, initial weight) and (W2, weight after desiccation).
Percentage moisture loss: The patches were weighed accurately and considered as initial weight and kept in a desiccators containing anhydrous calcium chloride. After 5 days, the patches were taken out and weighed considered as final weight. The percentage of moisture loss was calculated as the difference between final and initial weight with respect to initial weight.
In vitro drug release: The modified glass diffusion cell was used as diffusion apparatus, cellophane membrane attached to one of its end which was soaked previously in phosphate buffer pH 7.5 for 24 hrs. [15]. The donor compartment i.e. glass tube and receptor compartment was adjusted in such a way that membrane just touched the surface of receptor medium. The patches were cut in to the dimensions of 1 cm2 and placed on cellophane membrane. The temperature of receptor medium was maintained by circulating water bath at 37ºC and was kept continuously stirred at 60 rpm on magnetic stirrer. The sink condition was maintained by withdrawing 5 ml aliquots of samples from the receptor medium at predetermined time intervals and replaced with equal volume of fresh buffer. Samples were analysed with The HPLC system consisted UVvisible detector (SPD-10A). A filtrate of 15 μL was injected into HPLC system containing C18 reverse phase column using mobile phase of acetonitrile and phosphate buffer pH 7.4 (95:5 V/V). The flow rate was 1.0 ml/min with detection at 220 nm.
Selection of population for trial: The human ethical committee of Department of Anesthesiology, The second people’s hospital of Jiaozuo; China had approved the protocol (ANE/0504/Jiaozuo) for present trial. Healthy human volunteers were selected and made aware about the nature, structure and intention of the study and written informed consent was obtained from them. The trial was a controlled, randomized containing group of 10 patients in each group (n=10).
Application of LidoSite™ Controller system and patches to volunteers: The LidoSite™ Controller was used as per the instructions given in product leaflet. The LidoSite™ Controller is designed with a non-replaceable battery that provides up to 99 drug applications at 1.77 mA for 10 minutes (17.7 mAmin). Refer to the “LidoSite™ Topical System Instructions” for proper system operation. LidoSite™ System was applied only by a health care practitioner in a health care setting. The intended application site was made hairless for proper patch adhesion. The LidoSite™ Patch reservoirs were kept in complete contact with the skin during treatment. Therefore, restricting motion was recommended for those application sites where movement could release the patch from the skin.
All the patients were administered with their respective test or control samples at same time using same experimental conditions. The effects were measured in terms of end points. Before starting the treatment the patients were given neither topical anesthesia nor any premedication like NSAID’s. The IP setup was established using patch and electrodes and treatments was started whose results are measured in terms of end points. IP was applied for and up to period of 10 min.
Efficacy end points: The efficacy end points were measured at 0, 5,10,15,20 and 30 minutes after treatment. The parameters like warm sensation, cool sensation and hot pain were considered for the measurement of duration of anaesthesia. Depth of anaesthesia was also determined by measurement of pain sensation using 27 gauge needles. The blood samples were collected at 0 min, 10 min and 30 min to determine the absorption rate of Lignocaine [16].
Thermal threshold testing: The thermal thresholds (3) were established in the central portion of the treated area; warm, cool and hot pain. The order of stimuli was kept same as it progresses from lowest to highest stimulus i.e. lowest to highest. The meander electrode was used to measure warm and cool sensations which consist of electrodes of alternating polarity and gap is filled with insulating material. The temperature of the thermode was either increased or decreased at a rate of 1.0°C/second, depending on the direction of current flow through the device. The patient holds a switch that is pressed at the first sensation of warmth or cold; pressing the switch reverses the temperature change, returning to a neutral temperature of 32°C. Warm pain measurements also used the Thermal Sensory Analyzer, but the end point is pain instead of temperature change sensation [17,18].
Depth of anesthesia (pain intensity score measurement): The test was performed in blindfold subjects by mounting sterile 27-gauge short needle perpendicular to the skin. The needle was pinned gently in one direction with controlled and continuous movement to targeted site. The needle was pinned in slowly and smoothly. The intensity of pain should be measured at first sensation of pain felt by subject and recorded as Visual analogue scales (VAS) read out as ‘no pain’ (0) to ‘worst pain’ (10). The needle must be removed at first sensation of pain. Needle should be changed each time for each individual subject. To avoid human error the same investigator must perform all the measurements for all groups.
The same end point was measured using depth of needle insertion. [19] After completion of treatment the sterile 27 gauge short bevel needle was inserted into the forearm of the patient and thumb roll knob on gauge scale was rotated downwards which makes needle to be inserted in downwards. The readings were recorded in increments of 0.001 mm. The needle was removed immediately after the first sensation of pain and reading was noted as shown on readout device.
Skin effects: The surface of the skin was examines visually for any observable signs such as redness, swelling etc.
Data analysis: The statistical experimental data were analyzed using the Design-Expert® Software. Effect concentration of epinephrine on depth of anesthesia, cool sensation, warm sensation and hot pain sensation was determined using ANOVA test.
Results
Both the electrolyte and drug reservoir patches were prepared successfully by casting solvent evaporation method and evaluated for their physical characteristics as shown in Table 1. All the patches were found to be smooth in appearance and showed good surface characteristics. The patches were semi-transparent in nature with thickness in range of 0.03 ± 0.004 to 0.05 ± 0.006 mm. The uniformity of drug content ranges from 94.79 ± 1.45 to 98.45 ± 0.06%. The tensile strength was calculated as 0.42 ± 0.006 to 0.58 ± 0.002 kg/cm2. The average weight of the patches ranged from 0.15 ± 0.2 to 0.17 ± 0.18 gm. The folding endurance was found to be 145.21 ± 0.2 to 175 ± 0.29.The moisture content of patches were found to be 1.43 ± 0.23% to 2.97 ± 0.23%. In vitro release performed in Phosphate buffer pH 7.4 was found to be 45.56% to 87.23 ± 0.24 % in 24 hr.
| Batch | Appearance | Thickness (mm) | Wt variation (g) |
Folding endurance | Drug content uniformity (%) | Tensile strength (Kg/cm2) | Moisture absorption (%) | Moisture loss (%) |
|---|---|---|---|---|---|---|---|---|
| AT1 | T | 0.030 ±0.1 | 0.167 ± 1.0 | 146.2 ± 2.2 | 94.21 ± 0.9 | 0.42 ± 0.1 | 1.4 ± 0.25 | 1.2 ± 0.23 |
| AT2 | S | 0.045 ± 0.2 | 0.159 ± 1.2 | 163.3 ± 3.1 | 97.37 ± 0.9 | 0.48 ± 0.2 | 1.8 ± 0.21 | 2.3 ± 0.27 |
| AC | R | 0.051 ±0.1 | 0.169 ± 1.5 | 169.2 ± 1.9 | 98.13 ± 0.9 | 0.58 ± 0.1 | 2.9 ± 0.22 | 1.2 ± 0.31 |
Table 1: Evaluation and Physical Characterization of patches.
Thirty participants (15 females/15 males) entered the study. Patient characteristics and descriptive details were enlisted in Table 2. There was no significant statistical difference between mean age (p=0.54), the female: male ratio (p=0.5), weight (p=0.71) and height (p=0.68) in each treatment group.
| AT1 | AT2 | AC | |
| N | 10 | 10 | 10 |
| Age (yrs) | 49 (30-72) | 51 (28-71) | 50 (27-72) |
| Weight (kg) | 56-89 | 59-85 | 57-86 |
| Height (cm) | 152-185 | 154-187 | 155-189 |
| Ratio Female: Males | 1:1 | 1:1 | 1:1 |
Table 2: Demographic data of patients entering the trial and receiving AT1, AT2, and AC as local anaesthetic.
Local skin effects
All the subjects who underwent the IP treatment experienced the very mild type of skin irritation which was completely subdued in 2-5 min after completion of treatment was observed. No patient entered in trial reported the swelling, itching or edema.
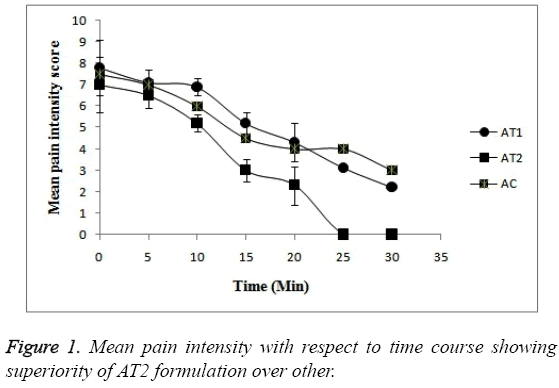
Pain intensity score measurement: The time courses of mean pain intensity after the treatment to all treatment groups with AT1, AT2 and AC are shown in Figure 1 and data in Table 3. The post treatment pain intensity of AC was numerically found to be much greater just after treatment i.e. at 0 hr when compared with the treatment groups AT1 and AT2. The pain was found to be subdued as time passes in all 3 formulations. In case of all 3 formulation groups the intensity of pain at baseline or at 0 hr was found to be numerically same but the as time passes the pain patients administered with AT2 formulation was relieved more (p<0.05). In fact after 20 min no patient felt the pain at all. Whereas in case of AT1 and AC formulation the intensity of pain is lowered but the score was less a when compared with AT2 formulation. The patients felt the pain even after 30 min in case of AT1 and AC formulations. So, this statistical difference in pain scores proves the superiority of AT2 formulation over other (p<0.05).
| Time (min) | Mean Pain Intensity Score (VAS 0-10) | ||
| AT1 | AT2 | AC | |
| 0 | 7.3 | 7 | 9 |
| 5 | 7.1 | 6.5 | 7 |
| 10 | 6.9 | 5.2 | 6 |
| 15 | 5.2 | 3 | 4.5 |
| 20 | 4.3 | 2.3 | 4 |
| 25 | 3.1 | 0 | 4 |
| 30 | 2.2 | 0 | 6 |
Table 3: Mean pain intensity score assessed by patients on VAS after treatment with each test formulation.
End point determination: Comparison of baseline values or values at 0 time course indicate that there is no significant difference between treatment condition for any end point i.e. for each end point the response at 0 hr is nearby same for all treatment groups. For assessment of effect of concentration of Epinephrine on Lignocaine the subjects were tested under control (AC), AT1, AT2 condition. As depicted in Table 3 the time dependent effects of all end points were studied for all mean pain intensity.
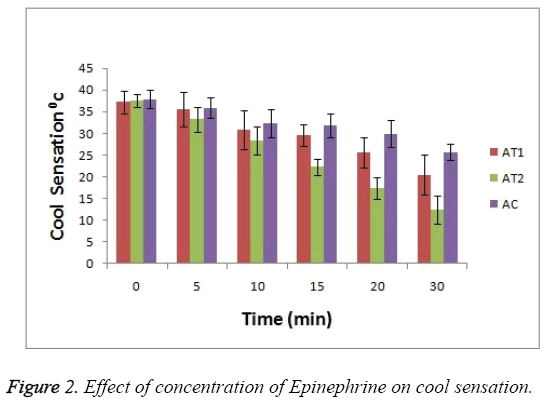
For cool sensation as it can be seen in Figure 2 that at 0 hr there was observance of no significant difference between 3 test groups but as the treatment time passes the ability of AT2 formulation to combat the cool sensation increases i.e. anaesthetic effect increases (p<0.05). This enhanced effect help to reduce the cool sensation. This activity is seen increased in case of AT2 test group as compare to AT1 and AC.
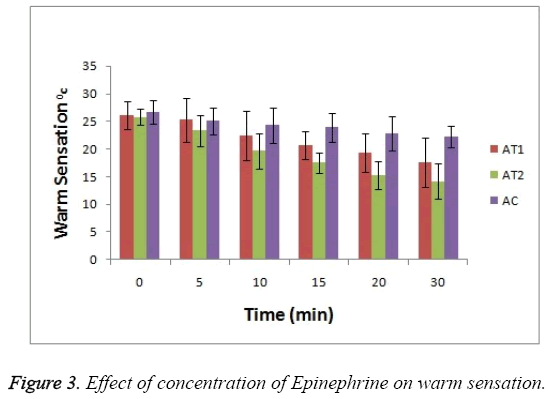
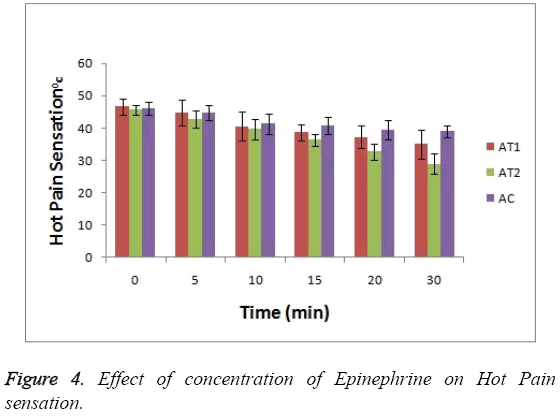
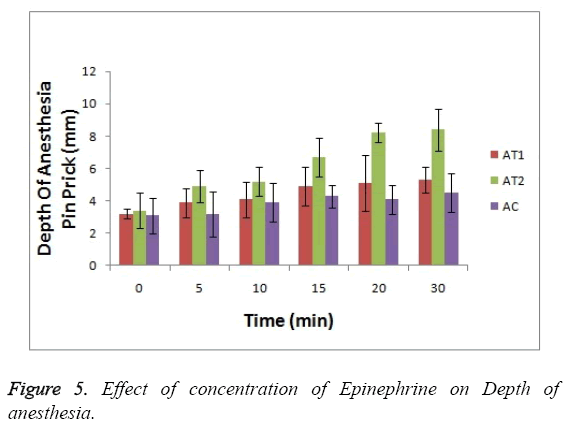
As shown in Figure 3 the baseline or at 0 hr the difference in sensation for all groups is less but it increases as the treatment proceeds. Formulation AT2 found to be superior in decreasing the warm sensation as compare to AT1 and AC (p<0.05). Figure 4 Depicts the effect of epinephrine and Lignocaine concentration on hot pain. As seen in Figure 4 the baseline values for all the groups are almost same. As the time passes from 0 min to 30 min the hot pain sensation decreases sharply for group AT2 while it shows less reduction in group AT1 and AC as compare to AT2. The effect on Depth of anesthesia can be seen in Figure 5. It measures the distance in mm upto which the needle can be inserted. From Figure 5 one can clearly state that distance of needle prick is highest for AT2 group i.e patients are more anesthetized in this group which indicates that depth of anesthesia is more for AT2 test group than others (p<0.05).
Discussion
All the subjects entered the trial were co-operative i.e. all patients completed the treatment. No patient from the selected population consumed alcohol during the entire trial period. Zero no. of patients had taken any analgesic medication. All the patients returned their CRF’s with complete filled information. In the present study, all patients used VAS i.e. vertically oriented scale comprising of 10 digits indicating the subjective pain intensity during total study/observation period i.e. 30 min. Scott J et al. had previously reported the successful use of VAS for comparison of local anesthetic in various preparations and/or surgical procedures. The results produced by VAS system has been compared with other pain scales which confirms the superiority of VAS [20].
The current investigation focuses on dose response relationship and effect of concentration of Epinephrine on Anaesthetic activity of Lignocaine. The results obtained from current investigation suggest that adrenaline if given exogenously possesses pain reducing capacity when co-administered with Lignocaine. As stated earlier the sympathomimetic amine exerts its action via interaction with adrenergic receptors. They are thought to be delaying absorption velocity of local anesthetic from the site of injection. The physical responses obtained from patients (like inflammation) involves complex set of cellular reactions which may be biochemical or cellular. Several researchers had made hypothesis regarding the effect of adrenaline on various local anesthetics. It can be possible that direct receptor mediated pharmacodynamic effects of adrenaline contribute to the soothening effect (i.e. reduction in pain intensity) of adrenaline.
The study undertaken here was controlled and randomized but the volume of local anesthetic kept constant and concentration of adrenaline kept varying for different treatment groups. The duration of treatment or treatment period was kept constant (or allowed for minimum variation in time) from different treatment groups. The physical responses obtained from the patients in present trial showed variation and distinct patterns of physical responses. The patients in the treatment group without adrenaline (AC) shows the observation of highest pain intensity the time course as there would be no effect of either adrenaline or Lignocaine itself. The initial mean pain intensity observed for treatment group AT2 approximately shows same level of pain intensity as AT1 or AC but rapidly decreases as the time for treatment passes. When compared to treatment group AT2, the AT1 and AC comparatively show less lowering of pain intensity.
The study suggest that the treatment group AT1 and AC initially produces nearly similar degree of local anesthesia as AT2 for warm sensation and hot pain threshold but the depth of anesthesia produced by AT2 becomes of greater depth as treatment time passes. The effect on cool sensation is also more for AT2 than for AT1 and AC. Like for skin temperature above 34ºC and below 32ºC produces the sensation of warmth and coolness respectively. Wallace M. et al had stated that cool sensations are results of response by small myelinated fibers while warmth and pain sensations are due to small unmyelinated fibres. The study undertaken here supports the fact that myelinated fibers are more sensitive to local anesthetic blockade as cool threshold [7].
So, it can be believed that effect of treatment group AT2 which is significantly higher than other treatment groups i.e. AT1 and AC confirms the fact that co-administration of epinephrine with local anesthetic Lignocaine significantly increases its anaesthetic activity in concentration or dose dependent manner. So, we can state that with increasing concentration of epinephrine increases anesthetic activity of Lignocaine. The total physical responses obtained in AT2 group are of relatively low intensity.
Conclusion
High epinephrine concentration combined with Lignocaine offers significant advantage for pain relief. As concentration of Epinephrine increases the pain relieving effect also increases. So, from the above investigation it can be concluded that coadministration of epinephrine with Lignocaine possesses pain relieving potential which influence the physical responses like cool, warm threshold and hot pain.
References
- Tyle P. Iontophoretic devices for drug delivery, American Cyanamid company, New Jersy.1986; 318-326.
- Sachan R, Bajpai M. Transdermal drug delivery system: a review, Int. J. of Res. & Dev. in Pharm. & Life Sci. 2013; 3:748-765.
- Jett WE. Three Generations: The Past, Present, and Future of Transdermal Drug Delivery Systems.
- Shingade GM, Aamer Q, Sabale PM, Grampurohit ND, Gadhave MV. Review on: recent trend on transdermal drug delivery system. Jof Drug Delivery & Therapeutics. 2012; 1:66-75.
- Prausnitz MR. Reversible skin permeabilization for transdermal delivery of macromolecules, Crit rev Therap Drug carrier Syst 1997;14:455-483.
- Hofmann GA, Rustrum WV, Suder KS. Electro-incorporation of microcarriers as a method for transdermal delivery of large molecules. Bioelectrochem Bioenerg.1995;38:209-222.
- Wallace SM, Ridgeway B, Jun E. Topical delivery of Lodocaine in healthy volunteers by Electroporation, Electroincorporation, or Iontoporesis: An evaluation of skin Anesthesia. 2001;26: 229-238.
- Patel D, Chaudhary SA. Transdermal Drug Delivery System: A Review, The pharma innovation. 2012; 1:66-75.
- LIDOSITE- lidocaine hydrochloride and epinephrine bitartrate patch B. Braun Medical, Inc.
- Rawat S, Vengurlekar S, Rakesh B, Jain S. Transdermal Delivery by Iontophoresis, Indian J Pharm Sci., 2008;70: 5-10.
- William T, Zempsky MD, Anand K. J. S. Lidocaineiontophoresis for topical anesthesia before intravenous line placement in children. The journal of pediatrics. 1998;12:1061-1063.
- Niemi G, Breivik H. Epinephrine Markedly Improves Thoracic Epidural Analgesia Produced by a Small-Dose Infusion of Ropivacaine, Fentanyl, and Epinephrine After Major Thoracic or Abdominal Surgery: A Randomized, Double-Blinded Crossover Study With and Without Epinephrine. AnesthAnalg. 2002;94:1598-1605.
- Gaumann D, Forster M. Comparison Between Clonidine and Epinephrine Admixture to Lidocaine in Brachial Plexus Block, Regional anesthesia and pain management anesthanalg; 1992;7569-7574.
- Demiana I, Nesseem SF, Eid SS, El-Houseny. Development of novel transdermal self-adhesive films for tenoxicam, an anti-inflammatory drug. Life Sciences.2011;89: 430-438.http://www.accessdata.fda.gov/scripts/CDER/dissolution/dsp_SearchResults_Dissolutions.cfm
- Christopher M, Bernards DJ. Effect of Epinephrine on Lidocaine Clearance In Vivo, Anesthesiology, 1999; 91:962–968.
- Catherine J, Sinnott LP. On the Mechanism by Which Epinephrine Potentiates Lidocaine’s Peripheral Nerve Block. 2003; 98:181-188.
- Yarnitsky D, Ochoa JL. Warm and cold specific somatosensory systems. Psychophysical thresholds, reaction times and peripheral conduction velocities. Brain. 1991; 14:1819-1826.
- Gasser HS, Erlanger J. The role of fiber size in the establishment of nerve block by pressure or cocaine. Am J Physiol 1929; 88:581-591.
- Gissen AJ, Covino BG, Gregust J. Differential sensitivities of mammalian nerve fibers to local anesthetic agents. Anesthesiology. 1985; 53:467-474.
- Scott J, Huskisson EC. Vertical or horizontal visual analogue scales. Ann Rheum Dis 1979; 38:560.