ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 2
In vitro and in vivo biological screening of kefiran polysaccharide produced by Lactobacillus kefiranofaciens
1Department of Zoology, Faculty of Science, King Saud University, Riyadh, Saudi Arabia
2Department of Natural and Microbial Products, Pharmaceutical Industries Division, National Research Centre, Egypt
3Institute of Bio Product Development, University Teknologi Malaysia, Malaysia
4Department of Bioprocess Development, City for Scientific Research and Technology Applications (CSAT), New Burg Al Arab, Alexandria, Egypt
5Department of Biochemistry, Science Faculty of Girls, King Abdulaziz University, Saudi Arabia
Accepted date: June 7, 2016
Kefiran is a functional fermented milk product traditionally used for its beneficial probiotic properties. It exhibits antimicrobial, antioxidant, anti-inflammatory anticancer and different health promoting characteristics. Although kefiran showed potential effects against many cancer cell lines, little information is present in the literature on its effect against cervical and hepatocellular carcinoma as well as on zebrafish embryos. The study aimed at investigating the cytotoxicity (in human cervical and hepatocellular carcinoma cell lines) and developmental toxicity (in zebrafish embryos) of kefiran produced by the fermentation of Lactobacillus kefiranofaciens. Cervical and hepatocellular cancer cells were exposed to serial concentrations of kefiran to evaluate its cytotoxic activities. Further biological effects of kefiran on the mortality and developmental abnormalities of zebrafish embryos were investigated. Results showed that kefiran significantly affected the viability of both tested cancer cell lines in a dose-dependent manner with IC50 values of 358.8 ± 1.65 and 413.5 ± 1.05 μg/ml for HeLa and HepG2 cells, respectively. Furthermore, kefiran adversely affected the morphological characteristics of the cells. Kefiran extract was much safer for zebrafish embryos and no mortality was observed up to 100 μg/ml, whereas the LC50 value (≥ 279.76 μg/ml) was also very high. Moreover, no developmental toxicity was observed up to 100 μg/ml concentration. Conclusively, microbially-produced kefiran showed anticancer properties in two tested human cancer cells, while its safer profiles in animals (zebrafish embryos) poses it as potential anticancer agent which does not affect normal tissue growth.
Keywords
Anticancer, HeLa, HepG2, Kefiran, Polysaccharides, Zebrafish embryos.
Introduction
Kefiran is a water-soluble exopolysaccharide produced by L. kefiranofaciens, and constitutes the matrix of the kefir grains, a fermented milk product traditionally consumed in eastern European countries [1,2]. Kefir constitutes a microbial consortium of lactic acid bacteria (Lactobacillus, Lactococcus, Streptococcus and Leuconostoc) and acetic acid bacteria (Acetobacter) symbiotically growing with different yeast species (Saccharomyces, Candida and Kluyveromyces) [3]. Kefir has many potential applications as a functional food component and exhibited numerous probiotic characteristics. Also, kefir helps in the stabilization of hypertension, decreases the levels of serum cholesterol [4,5]. Kefiran is a heteropolysaccharide having a ratio of 1:1 of glucose and galactose and is mainly produced by the lactic acid bacteria and yeasts present in the kefir grains [6-8]. In addition, kefiran has been recognized by the US Food and Drug Administration as GRAS product (Generally Regarded as Safe), and found many potential applications in food as well as in pharmaceutical industries [2]. Moreover, kefiran has been reported to possess antibacterial, antifungal, antioxidant and anti-inflammatory properties, and has been used to lower serum cholesterol level and to modulate the immune system [9-12]. Recently, kefiran showed a gastro protective effect on ulcers induced in irradiated rats [13], and was reported to inhibit the growth of several cancer types; i.e. Ehrlich carcinoma, Lewis lung carcinoma and breast carcinoma [14,15].
Zebrafish (Danio rerio) are proven in vivo model which is best suited for drug discovery and screening of small molecules [16,17]. The embryos of zebrafish are transparent and development occurs externally, enabling an easy and thorough assessment of drug effects on internal organs in live organisms. It is known that zebrafish are used at various stages of the drug discovery process as a cost-effective alternative to some mammalian models [18-20]. Zebrafish embryogenesis is very rapid, with the entire body plan established by 24 hours postfertilization (hpf). The zebrafish embryo is also an attractive model for studying neurogenesis as it is a vertebrate with the conserved organization of common tissues including the brain and the spinal cord. The neurogenesis starts around 10 hours post fertilization (hpf), synaptogenesis and the first behaviours around 18 hpf [21].
Little information is present in the literature on the biological activities of microbially produced kefiran on human cervical and hepatocellular carcinoma as well as in zebrafish embryos. Therefore, the present study was designed to evaluate the possible cytotoxic properties of kefiran produced by L. kefiranofaciens in vitro in human cervical cancer cells (HeLa) and human hepatocellular carcinoma cells (HepG2) and in vivo developmental toxicity in zebrafish embryos.
Materials and Methods
Materials
Unless otherwise stated, all chemicals, reagents and disposables were of cell culture grade and were purchased from Sigma-Aldrich Chemical Company, St. Louis, USA.
Preparation of kefiran polysaccharide
L. kefiranofaciens ATCC 8007 cells were used to produce the kefiran polysaccharide according to our previously published work [1,2]. Cells were grown in 250 ml Erlenmeyer shakeflasks for 72 h at 30ºC and 200 rpm on a rotary shaker (Innova 4080, New Brunswick Scientific, NJ, USA). The optimized cultivation medium contained (g/L): lactose, 50.0; yeast extract, 12.0; KH2PO4, 0.25; sodium acetate, 5.0; Triammonium citrate, 2.0; MgSO4.7H2O, 0.2; MnSO4.5H2O, 0.05. The pH of the medium was adjusted to 5.5. Lactose was sterilized separately at 100ºC for 20 min and was added to the cultivation medium before inoculation.
The method Piermaria et al. [22] was adapted to recover and determine the extracellularly produced kefiran. Briefly, the culture supernatant was incubated overnight with an equal volume of cold absolute ethanol at 4ºC in order to precipitate kefiran. The mixture was then centrifuged at 9000 rpm for 15 min, and then the obtained precipitate was dissolved in hot distilled water and back-precipitated with ethanol. The last step was repeated three times to obtain kefiran in a pure form. The finally precipitated pure kefiran was collected, dried at 65ºC for 48 h, and then stored for further steps.
The purified kefiran was dissolved in DMSO to prepare the stock solution (1 mg/ml), which was used to prepare a series of different working solutions (0-1000 μg/ml) using DMEM medium. The working solutions were aseptically filtered using 0.22 μm sterile syringe filters (Millipore, USA).
Cell lines and cultivation conditions
Human cervical cancer (HeLa) and human hepatocellular carcinoma (HepG2) cells were obtained from Sigma-Aldrich Chemical Company, St. Louis, USA. Cells were grown on DMEM medium containing foetal bovine serum (10%), penicillin/streptomycin solution (100x, 1%) and NaHCO3 (3.6 g/L). According to standard cell culture protocols, cells were routinely sub-cultured and in a humidified CO2 incubator (ShelLab, USA) at 5% CO2, 37ºC and 95% humidity. Viable cell concentration as well as cell viability were assessed using the Trypan blue exclusion method [23,24].
Cytotoxicity assay
Standard MTT (3- (4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyl) cytotoxicity assay [25] was used to determine the percentage of cell viability. Cells were firstly treated with trypsin, washed and then resuspended. Only cells having viability scores higher than 95% were used to perform the cytotoxicity assays. 96 well culture plates were inoculated with cells to reach a concentration of 104 cells/100 μl/well, and were then allowed to grow for 24 h. The consumed medium was then aspirated and replaced with fresh preparations containing different concentrations of kefiran working solutions, and the cells were then grown for another 24 h. DMSA only at a final concentration (≤ 0.5%) served as the control. Firstly, the plates were examined for morphological changes using an inverted contrast microscope (Nikon Eclipse T500, Japan, and 10x). Cells were then treated with MTT (10 μl, 5 mg/ml in PBS) for 4 h, and the resulting formazan crystals were dissolved with 200 μl of DMSO. The absorbance was read at 550 nm using a micro plate reader (Thermo Scientific, USA). Cell viability was calculated as a percentage of the control value. The concentration resulting in 50% inhibition of cell growth was referred to the IC50 value, and was determined from the linear regression of the calibration curve.
Zebrafish embryos
Wild type zebrafish (AB/Tuebingen TAB-14) and transgenic TG (Fli;1:EGFP) y1 [16] were obtained from the zebrafish International Resource Centre (ZIRC University of Oregon, Oregon, USA) and maintained in the animal facility at Bio products research chair, Department of Zoology at King Saud University. The adult tropical zebrafish were kept under the standard laboratory conditions of 28.5ºC on a 14-h light/ 10-h dark photoperiod in fresh water (FW) which consists of reverses osmosis water supplemented with a commercially available salt solution (0.6% Instant Ocean) according to the standard guidelines that are described in the literature [17]. All experiments were carried out in accordance with the National and International animal use guidelines and were in accordance with the ethical guidelines of the College of Science, King Saud University.
Animal treatment
The wild type (AB Tubingen) and transgenic Tg (fli-1:EGFP) zebrafish embryos were obtained by natural pairwise mating and raised up to the shield stage (6 hours post fertilization). The extracted kefiran was re-suspended in the cell culture grade DMSO (D8418 Sigma LLC., St. Louis, USA) to prepare a stock concentration of 20 mM. The synchronized embryos (all embryos were at the same stage of development) were treated with a serial dilution of kefiran in order to assess both the toxicity and developmental abnormalities. Approximately thirty (30) embryos were placed in sterile 60 mm Petri dishes that contained 10 ml of the embryo medium (5.0 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl, 0.33 mM MgSO4) at the desired concentration of kefiran. The 1% (v/v) DMSO treated embryos served as controls. The embryos were then incubated overnight at 28ºC in an air incubator. From the second day onward, the embryo medium containing the kefiran was changed daily. Three biological replicate trials (each clutch of the embryo was from different adult pair of fish) were carried out for each experiment [20].
Microscopic examination
All images were acquired using fluorescent stereo microscope Olympus ZX12 with DP72 camera using CellSens standard software (Olympus Capital Holdings Asia Pte Ltd. Singapore).
Statistical analysis
Data were analysed using SPSS 9.0, and the obtained results are represented as mean ± SD of three experiments. One-way ANOVA analysis of variance and student t-Test was used to compare between different experimental groups and data were considered statistically significant for P values less than 0.05.
Results
Evaluation of in vitro cytotoxicity of kefiran polysaccharides
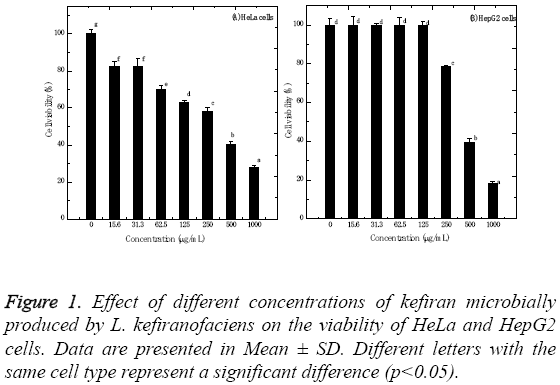
The kefiran polysaccharides produced by L. kefiranofaciens were evaluated for its in vitro cytotoxic properties against HeLa and HepG2 cells using standard MTT assay. Figure 1 shows the cytotoxic effect of kefiran, which was dose- and cell type-dependent. The IC50 values for HeLa and HepG2 cells were 358.8 ± 1.65 and 413.5 ± 1.05 μg/ml, respectively. Increasing kefiran concentration significantly increased the cytotoxicity of kefiran to HeLa and HepG2 cells (p<0.001). The highest kefiran concentration (1 mg/ml) significantly decreased cell viability by about 72.25 and 81.85% recording 27.75 ± 1.31 and 18.15 ± 0.88% for HeLa and HepG2 cells, respectively. Moreover, the results showed that HeLa cells were not significantly affected when being tested with 15.6 or 31.3 and 125 μg/ml of kefiran. On the other hand, HepG2 cells showed no significant changes when treated with kefiran concentrations ranging from 0.0 to 125 μg/ml.
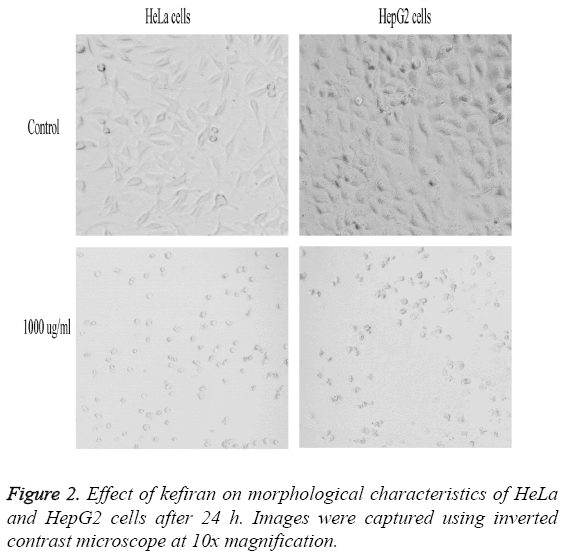
Figure 2 represents the effect of variable concentrations of kefiran on the morphological characteristics of both HeLa and HepG2 cells compared to control untreated cells. Increasing kefiran concentration drastically affected the morphological characteristics of both cell lines, with the maximal effect observed upon using the highest kefiran concentration (1 mg/ ml). Upon increasing kefiran concentration, cells started to shrink, lost their adherence capacity and finally started to float in the cultivation flask. The maximal kefiran concentration resulted in the complete rounding-up of the cells with the formation of vacuoles leading finally to their death.
In vivo cytotoxic effects of kefiran polysaccharide in zebrafish embryos
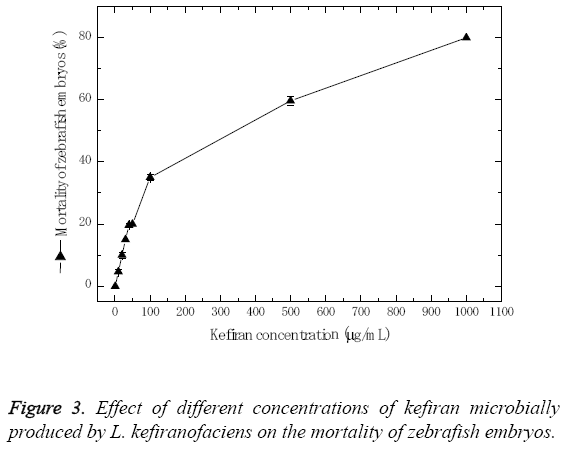
In order to determine the in vivo developmental toxicity of kefiran in zebrafish embryos, the embryos were exposed to serial dilution of kefiran ranging from 1.0 to 1000 μg/ml. The zebrafish embryos responded dose dependently as depicted in Figure 3. Results showed that mortality percentage increased with the increase of kefiran concentration, where the maximum concentration of kefiran (1 mg/ml) resulted in about 80% mortality, and the LD50 concentration was ≤ 279.76 μg/ml.
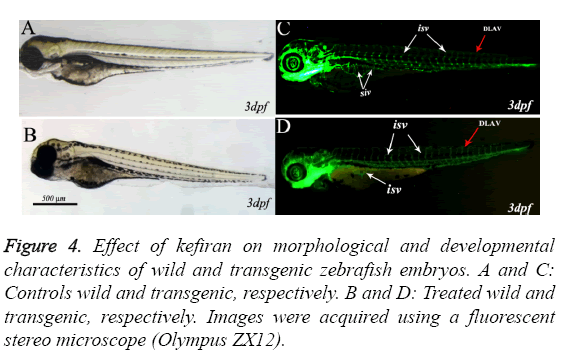
Figures 4A-4D represent images taken during the assessment of developmental abnormalities in zebrafish embryos induced by kefiran (at 10-30 μg/ml) in comparison with mock treated embryos at the same stage. The zebrafish embryos hatched normally at 48-52 hpf and started to swim normally directly after being hatched. The brain has developed normally in all treated embryos (Figure 4B) and all the brain structures (fore brain FB, mid brain MB and hind brain HB ) along with mid brain hind brain boundary MHB developed normally and there was no obvious abnormality in brain formation compared to control (Figure 4B).
Figure 4: Effect of kefiran on morphological and developmental characteristics of wild and transgenic zebrafish embryos. A and C: Controls wild and transgenic, respectively. B and D: Treated wild and transgenic, respectively. Images were acquired using a fluorescent stereo microscope (Olympus ZX12).
The zebrafish transgenic line TG (fli-1; EGFP), expressing the enhanced green fluorescent protein (EGFP) in endothelial cells under the promoter of fli1 [16], is routinely used to screen antiangiognesis molecules. This transgenic zebrafish line was also used in the current work to validate whether kefiran disrupted the formation of angiogenic blood vessels.
Figure 4 shows representative live images of 3 dpf transgenic zebrafish embryos control (C) and treated with kefiran (D). In control-as well as kefiran-treated embryos, it can be clearly observed that the intersegmental blood vessels (ISV) sprouted from the dorsal aorta (DA) and extended along with the boundaries of somites and connected to the dorsal longitudinal anastomotic vessel (DLAV, red arrow).
Similarly, the second angiogenic blood vessels, which form the sub intestinal vein (SIV white arrows) and are composed of an arcade of 10 to 12 vessels arranged as a basket like structure on the yolk, developed normally in treated embryos at 72 hpf. Therefore, it can be concluded that treating zebrafish embryos at the tested concentration did not induce any abnormalities during embryonic development.
Discussion
Kefiran is a functional fermented milk product with a wide range of probiotic properties. Regardless of its nutritional value, kefiran exhibits different biological activities [9,10,26]. Recently, many studies were conducted to evaluate the cytotoxic activities of kefiran against different cancer cell types and animal models. However, little information can be traced in the literature concerning studies on the evaluation of kefiran biological activities in vitro and in vivo on cervical as well as hepatocellular cancers and zebrafish embryos, respectively.
Kefiran exhibited a significant cytotoxic activity against both HeLa and HepG2 cell lines. Kefiran reduced the cell viability of HeLa and HepG2 cells up to 72.25 and 81.85%, respectively, upon applying the highest kefiran concentration (1 mg/ml). Additionally, the IC50 values recorded for HeLa and HepG2 cells were 358.8 ± 1.65 and 413.5 ± 1.05 μg/ml, respectively. Polysaccharides produced by microorganisms have been long used for their potential biological properties [27,28]. The obtained results are in good agreement with those reported earlier reporting anticancer activities of kefiran against Ehrlich, lung and breast carcinoma [15,29-31]. Recently, Maalouf et al. [32] obtained about 88.1 and 86.7% reduction in cell viabilities of CEM and Jurkat cells after 24 h of treatment with 60 μg/μL of kefir. However, they were not able to determine the IC50 values for kefir because of the high viscosity of kefir. The anticancer effect of kefiran can be attributed to the effect of the exopolysaccharide, i.e. kefiran, lentinan, viilian. β-1, 3-Glucans with 1, 6-glucopyranoside branching have been reported to produce effector activities in tumour synergic cell cytotoxicity [33,34]. Furthermore, our results showed that both tested cell lines responded differently to the kefiran polysaccharide. This can be explained by the fact that different types of cancer cells differ in their specificity and selectivity arising from differences in cell morphology and membrane structures [24,35,36].
The in vivo toxicity profile of kefiran on zebrafish embryos could be correlated with the in vitro cytotoxicity, with an LD50 value of 279.76 μg/ml. This could be considered as a very high concentration of a compound to affect the zebrafish embryos. Generally, the toxicity profile of active compounds lies within the range of 1-30 μg/ml. The transgenic zebrafish has been used for the evaluation of the antiangiogenic properties of newly synthesized compounds [20]. We also tested the effect of kefiran on zebrafish blood vessels formation. As shown in Figure 4, kefiran did not induce any abnormality in zebrafish angiogenic blood vessels formation and development. Moreover, we could not observe any developmental abnormality or teratogenic profile of Kefran in zebrafish embryos which mean that it turned out to be very safe in animals. Furthermore, the results concerning the effect of kefiran on the development of zebrafish embryos showed that kefiran has no obvious effect. Our results coincide with those reported by Kang et al. [37,38]. They evaluated the antioxidant activity of polysaccharides purified from Acanthopanax koreanum Nakai and aloe vera gel in zebrafish model, and found no effect on the survival rate of zebrafish embryos. Moreover, they reported a protective effect when the embryos were pre-treated with the polysaccharide prior to oxidative stress induction. Recently, Guven et al. [39] investigated the effect of kefir on spinal cord injury ischemia in rats. They concluded that kefir has a neuroprotective and anti-oxidant effects spinal cord ischemia and injury, where lactic acid bacteria produce bioactive peptides, which capture the reactive oxygen species and thus inhibit the formation of malondialdehyde, responsible for cell membrane damage mediated by lipid peroxidation.
Additionally, our results can be explained on the basis that the routine protocol depends on sub lethal concentrations to evaluate the developmental abnormalities in zebrafish embryos [19,20]. The sub lethal concentration of kefiran (10-100 μg/ml) did not induce any developmental abnormality which could be considered as safer in zebrafish embryos. The higher toxicity profile, which was observed with kefiran used in this study, could be attributed to the fermentation process as it was separated and extracted from a fermentation culture broth obtained. This in turn can explain the slightly higher data regarding cell toxicity and mortality of zebrafish embryos, in comparison to pure kefiran compounds used in previous studies.
Conclusion
The present investigation provides sufficient evidences that kefiran polysaccharide produced by L. kefiranofaciens showed a statistically significant level of anti-proliferative effect on human cervical cancer cells (HeLa) and human liver hepatocarcinoma cells (HepG2). Moreover, the anticancer profile was not only dose dependent, but also cancer cell type specific. Furthermore, kefiran did not induce any developmental toxicity in zebrafish embryos, at sub lethal concentrations. The anticancer profile specifically only in human cancer cells and safer behaviour of kefiran in zebrafish embryos warrants its potential anticancer properties. Furthermore, considering the lack of information on the effect of kefiran on the development of zebrafish embryos, these results can provide a preliminary platform for future research.
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group project "RGP-1435-047". The Authors are also thankful for Research Management Centre, University Technology Malaysia for funding the project entitled: Bioprocess optimization for efficient kefiran production by L. kefiranofaciens in semiindustrial scale. Vote No. Q.J130000.2609.06J04.
References
- Dailin DJ, Elsayed EA, Othman NZ, Malek RA, Ramil S, Sarmidi MR, Aziz R, Wadaan MA, El-Enshasy HA. Development of cultivation medium for high yield kefiran production by Lactobacillus kefiranociens. Inter J Pharm PharmSci 2015; 7: 159-163.
- Dailin DJ, Elsayed EA, Othman NZ, Malek RA, Phin HS, Aziz R, Wadaan MA, El-Enshasy HA. Bioprocess development for kefiran production by Lactobacillus kefiranofaciens in semi industrial scale bioreactor. Saudi J BiolSci 2015; 23: 495-502.
- De Vuyst L, De Vin F. Exopolysaccharides from lactic acid bacteria. In: Comprehensive glycol sciences. Elsevier UK 2007; 477-519.
- Cheirsilp B, Shoji H, Shimizu H, Shioya S. Interactions between Lactobacillus kefiranofaciens and Saccharomyces cerevisiae in mixed culture for kefiran production. J BiosciBioeng 2003; 96: 279-284.
- Maeda H, Zhu X, Omura K, Suzuki S, Kitamura S. Effects of an exopolysaccharide (kefiran) on lipids, blood pressure, blood glucose, and constipation. Biofactors 2004; 22: 197-200.
- Yokoi H, Watanabe T, Fuji Y. Isolation and characterization of polysaccharide-producing bacteria from kefir grains. Dairy Sci1990; 73: 1684-1689.
- Wang SY, Chen HC, Liu JR, Lin YC, Chen MJ. Identification of yeasts and evaluation of their distribution in Taiwanese Kefir and Viili starters. J Diary Sci 2008; 91: 3798-3805.
- La Riviere JWM, Kooiman P, Schmidt K. Kefiran, a novel polysaccharide produced in the kefir grain by Lactobacillus brevis. Arch Microbiol 1967; 59: 269-278.
- Rodrigues KL, Caputo LRG, Carvalho JCT, Evangelista J, Schneedorf JM. Antimicrobial and healing activity of kefir and kefiran extract. Int J Antim Agents 2005; 25: 404-408.
- Lee MY, Ahn KS, Kwon OK, Kim MJ, Kim MK, Lee IY, Oh SR, Lee HK. Anti-inflammatory and anti-allergic effects of kefir in a mouse asthma model. Immunobiology 2007; 212: 647-654.
- Huang Y, Wang X, Wang J, Wu F, Sui Y, Yang L,Wang Z. Lactobacillus plantarum strains as potential probiotic cultures with cholesterol-lowering activity. J Dairy Sci 2013; 96: 2746-2753.
- Güven A, Güven A, Gülmez M. The effect of kefir on the activities of GSH-Px, GST, CAT, GSH and LPO levels in carbon tetrachloride-induced mice tissues. J Vet Med B Infect Dis Vet Public Health 2003; 50: 412-416.
- Fahmy HA, Ismail AFM. Gastroprotective effect of kefir on ulcer induced in irradiated rats. J PhotochemPhotobiol B Biol 2015; 144: 85-93.
- Rizk S, Maalouf K, Baydoun E. The antiproliferative effect of kefir cell-free fraction on Hut-102 malignant T lymphocytes. Clin Lymphoma Myeloma 2009; 9: 198-203.
- LeBlanc A, Matar C, Farnworth E, Perdigon G. Study of cytokines involved in the prevention of a murine experimental breast cancer by kefir. Cytokine 2006; 34: 1-8.
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. DevBiol 2002; 248: 307-318.
- Westerfield M. edit. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio) 1995. University of Oregon Press, Oregon 1995.
- Parng C, Seng WL, Semino C, McGrath P. Zebrafish: a preclinical model for drug screening. Assay Drug DevTechnol 2002; 1: 41-48.
- Peterson RT, Link BA, Dowling JE, Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. ProcNatlAcadSci USA 2000; 97: 12965-12969.
- Farooq M, Abu Taha N, Butorac RR, Evans DA, Elzatahry AA, Elsayed EA, Wadaan MA, Al-Deyab S, Cowley AH. Biological screening of newly synthesized BIAN N-heterocyclic gold carbene complexes in zebrafish embryos. Int J MolSci 2015; 16: 24718-24731.
- Kabashi E, Champagne N, Brustein E, Drapeau P. In the swim of things: recent insights to neurogenetic disorders from zebrafish. Trends Genet 2010; 26: 373-381.
- Piermaria JA, Pinotti A, Garcia MA, Abraham AG. Films based on kefiran, an expopolysaccharide obtained from kefir grain: development and characterization. Food Hydrocolloids 2009; 23: 684-690.
- El-Enshasy HA, Abdeen A, Abdeen SH, Elsayed EA, El-Demellawy M, El-Shereef AA. Serum concentration effects on the kinetics and metabolism of HeLa cell growth and cell adaptability for successful proliferation in serum free medium. World ApplSci J 2009; 6: 608-615.
- Farooq, M, Hozzein, WN, Elsayed, EA, Taha, NA, Wadaan, MA. Identification of histone deacetylase I protein complexes in liver cancer cells. Asian Pac J Cancer Prev 2013; 14: 915-921.
- Elsayed EA, Sharaf-Eldin MA, Wadaan MA. In vitro evaluation of cytotoxic activities of essential oil from Moringaoleifera seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 cell lines. Asian Pacific J Cancer Preven 2015; 16: 4671-4675.
- Rodrigues KL, Caputo LRG, Cravalho JCT, Evangelista J, Schneedorf JM. Antimicrobial and healing activity of kefir and kefiran extract. Int J Antimicrob Agents 2005; 25: 404-408.
- El-Enshasy HA, Elsayed EA, Aziz R, Wadaan MA. Mushrooms and truffles: historical biofactoriesfor complementary medicine in Africa and in the Middle East. Evidence-Based ComplemenAltern Med 2013; 2013: 1-10.
- Elsayed EA, El-Enshasy H, Wadaan MA, Aziz R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediators of Inflamm 2014; 2014: 1-15.
- Chen C, Chan HM, Kubow S. Kefir extracts suppress in vitro proliferation of estrogen-dependent human breast cancer cells but not normal mammary epithelial cells. J Med Food 2007; 10: 416-422.
- Liu JR, Wang SY, Lin YY, Lin CW. Antitumor activity of milk kefir and soy milk kefir in tumor-bearing mice. Nutr Cancer 2002; 44: 182-187.
- Furukawa, N, Matsuoka A, Takahashi T, Yamanaka Y. Anti-metastatic effect of kefir grain components on Lewis lung carcinoma and highly metastatic B16 melanoma in mice. J AgricSci 2000; 45: 62-70.
- Maalouf K, Baydoun E, Rizk S. Kefir induces cell-cycle arrest and apoptosis in HTLV-1-negative malignant T-lymphocytes. Cancer Manag Res 2011; 3: 39-47.
- Adachi S. Lactic acid bacteria and the control of tumours. In: The lactic acid bacteria. Volume 1: The lactic acid bacteria in health and disease. Elsevier Sci UK 1992; 233-261.
- Yamada H, Kawaguchi N, Ohmori T, Takeshita Y, Taneya S. Structure and antitumor activity of an alkali-soluble polysacchardie from Cordycepsophioglossoides. Carbohydr Res 1984; 125: 83-91.
- Al-Salahi R, Elsayed EA, El Dib RA, Wadaan M, Ezzeldin E, Marzouk M. Synthesis, characterization and cytotoxicity evaluation of 5-hydrazono-[1,2,4]triazolo[1,5-a]quinazolines (Part I). Lat Am J Pharm 2016; 35: 58-65.
- Al-Salahi R, Elsayed EA, El Dib RA, Wadaan M, Ezzeldin E, Marzouk M. Cytotoxicity of new 5-hydrazono-[1,2,4]triazolo[1,5-a]quinazolines (Part II). Lat Am J Pharm 2016; 35: 66-73.
- Kang M-C, Kim S-Y, Kim YT, Kim E-A, Lee S-H, Ko S-C, Wijesinghe WA, Samarakoon KW, Kim YS, Cho JH, Jang HS, Jeon YJ. In vitro and in vivo antioxidant activities of polysaccharide purified from aloe vera (Aloe barbadensis) gel. Carbohydr Polymers 2014; 99: 365-371.
- Kang M-C, Kim S-Y, Kim E-A, Lee J-H, Kim Y-S, Yu S-K, Chae JB, Choe IH, Cho JH, Jeon YJ. Antioxidant activity of polysaccharide purified from AcanthopanaxkoreanumNakai stems in vitro and in vivo zebrafish model. Carbohydr Polymers 2015; 127: 38-46.
- Guven MD, Akman T, Yener AU, Sehitoglu MH, Yuksel Y, Cosar M. The neuroprotective effect of kefir on spinal cord ischemia/reperfusion injury on rats. J Korean NeurosurgSoc 2015; 57: 335-341.