ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 16
Immune status in patients with thyroid carcinoma and its correlation with clinicopathologic characteristics
1Cell Biology Laboratory, Jilin Province Institute of Cancer Prevention and Treatment, Jilin Cancer Hospital, Changchun, PR China
2Department of Internal Medicine, Southern Branch of Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, PR China
- *Corresponding Author:
- Shubin Li
Department of Internal Medicine
Southern Branch of Guang’anmen Hospital
China Academy of Chinese Medical Sciences
Beijing, PR China
Accepted date: July 31, 2017
Objective: To monitor the immune function of thyroid cancer patients and analyze its clinicopathological correlation.
Methods: The distribution of circulating lymphocyte subpopulations and serum cytokine (IFN-γ, TNF-α, TGF-β, and IL-10) levels in 59 Papillary Thyroid Cancer (PTC) patients, 33 goiter patients, and 50 healthy controls were measured by flow cytometry and ELISA.
Results: The percentages of CD3+ T lymphocytes and CD3+CD4+/CD3+CD8+ ratio in PTC patients decreased obviously. While the percentages of CD4+CD25+CD127low Tregs in PTC patients increased apparently, but it had no relationship with clinical characteristics. Serum TNF-α, IFN-γ, IL-10, but not TGF-β levels in PTC patients were significantly higher than that in healthy controls. The inclusion of these four cytokines improved the classification efficacy among the three groups.
Conclusion: It is apparent that the immune function is compromised in thyroid cancer patients. Combined detection of serum cytokines could help distinguish patients with thyroid disease and healthy individuals.
Keywords
Thyroid carcinoma, Nodular goiter, Cytokine, Immune function.
Introduction
Thyroid carcinoma is the most prevalent type of endocrine malignancy, and its incidence has been steadily rising in recent years. It can occur at any age, especially in young adults (30-40 y old) and women (about 75%). The initiation of thyroid cancer often appears gradually with no obvious symptoms. So if it can be diagnosed earlier, the therapeutic effects will usually be better [1].
Thyroid carcinoma can be subdivided into papillary, follicular, medullary, and anaplastic thyroid carcinoma. Their development is closely associated with immune dysfunction. There is accumulating evidence indicate that infiltration of immune cells in thyroid cancer lesions can predict prognosis [2,3]. Some thyroid diseases are known to be related to the development of thyroid cancer, such as goiters, which is the most common type of thyroid disease, including nodular goiter (primarily functional and morphological changes) and adenoma (benign tumor with high cancelation rate).
It is well known that inflammation is involved in the tumor development and suppression, and cytokines are the key mediators of inflammation. Several cytokines and chemokine’s produced by the tumor cells and by leukocytes and platelets associated with the tumor have been found to be able to maintain the invasive phenotype [4,5]. In 2016, Kobawala et al. reported serum levels of tumor necrosis factor-α (TNF-α), L-Selectin, and VCAM-1 were significantly higher in patients with both benign thyroid diseases and PTC as compared to the healthy individuals. They suggested that the circulating TNF-α levels could be used as a prognosticator for OS in PTC patients [6]. Stanciu et al. investigated the potential role of interleukin 4 (IL-4), interleukin 10 (IL-10) and high-sensitivity C-reactive protein (hs-CRP) as serum biomarkers of persistent/recurrent disease in PTC with/without Hashimoto’s Thyroiditis (HT). They found that increased levels of serum IL-4, IL-10 and hs- CRP were associated with persistent/recurrent disease in PTC and PTC+HT patients [7]. Discriminating which cytokines address the pathogenesis of the various diseases, including cancers, is a complex task. From this, derives the need to identify which cytokines might be suitable targets for fighting against cancer.
The aim of this study was to evaluate the alteration of peripheral blood lymphocyte subsets and serum TNF-α, interferon-γ (IFN-γ), IL-10, and transforming growth factor-β (TGF-β) levels in patients with goiters and thyroid carcinoma, and to correlate the results with clinicopathological parameters in thyroid cancer patients.
Materials and Methods
Patients and specimens
The present study was approved by the Ethics Committee of Jilin Cancer Hospital. Patients gave their informed consent before blood collection. The samples of thyroid cancer patients were selected based on two criteria: First hospitalized and no other autoimmune disease or other malignancies at the time of the investigation. From January 2016 to June 2016, peripheral blood samples of 59 cases of thyroid carcinoma (all were papillary thyroid cancer) and 33 cases of nodular goiters were collected prior to operation from Department of Thyroid and Neck Tumor, Jilin Cancer Hospital. 50 blood samples from healthy people were enrolled as control. From every patient and control, 2 ml of blood was withdrawn from the cubital vein. Serum samples were stored at -80°C.
Flow cytometry
Blood samples were analyzed by flow cytometry (FACS; Beckman Coulter) for phenotypic characterization of lymphocyte subsets. Cells were stained for 20 min at room temperature in the dark with CD45-FITC/CD4-RD1/CD8- ECD/CD3-PC5 and CD3-FITC/CD16+56-PE (Beckman Coulter). To determine the percentage of Treg cells, blood samples were stained with CD4-Percp, CD25-FITC, and CD127-PE (BD Biosciences-Pharmingen). OptiLyse@C Lysis Solution (Beckman Coulter) was intended for lysing red blood cells. Data were analyzed with the Expo32 ADC software (Beckman Coulter) [8].
ELISA
Measurements of serum concentrations of IFN-γ, TNF-α, IL-10, and TGF-β were performed by the ELISA method according to manufacturer’s instructions (Ebioscience).
Statistical analysis
Statistical analyses were performed with SPSS 23.0 (SPSS Inc., Chicago, USA) and GraphPad Prism 5 (GraphPad Inc., CA, USA). The significance level was defined as p<0.05. Differences in lymphocyte subsets and serum cytokine levels between groups were assessed by the student’s t-test or Mann- Whitney U-test. The Pearson correlation coefficient or Spearman’s rank correlation coefficient was used to describe the relationship between T cell subsets and serum cytokine levels and clinicopathologic characteristics. Fisher’s exact tests (or Chi-square tests) were used for all categorical variables. Receiver’s Operating Characteristic (ROC) curves were constructed to determine the discriminating efficacy of lymphocyte subsets and serum cytokine levels between healthy control and patients with thyroid diseases. The power calculations were performed by using G*Power 3.1.9.2 [9].
Results
Clinical patient characteristics
Characteristics of patients and the control group are summarized in Table 1.
| Characteristics | Control (n=50) | Goiters (n=33) | Thyroid cancer (n=59) | P value | |
|---|---|---|---|---|---|
| Control vs. Thyroid disease | Goiters vs. Thyroid cancer | ||||
| Sex | |||||
| Male | 20 (40%) | 10 (30%) | 10 (17%) | 0.034 | 0.22 |
| Female | 30 (60%) | 23 (70%) | 49 (83%) | ||
| Age (y) | |||||
| Median | 49 | 48 | 46 | 0.275 | 0.72 |
| Interquartile range | 44-56 | 35-57 | 42.25-56.75 | ||
| Range | 31-81 | 22-75 | 20-77 | ||
Table 1: Characteristics of patients and healthy individuals.
The distribution of circulating lymphocyte subpopulations of patients with PTC was different from that in goiters or control group
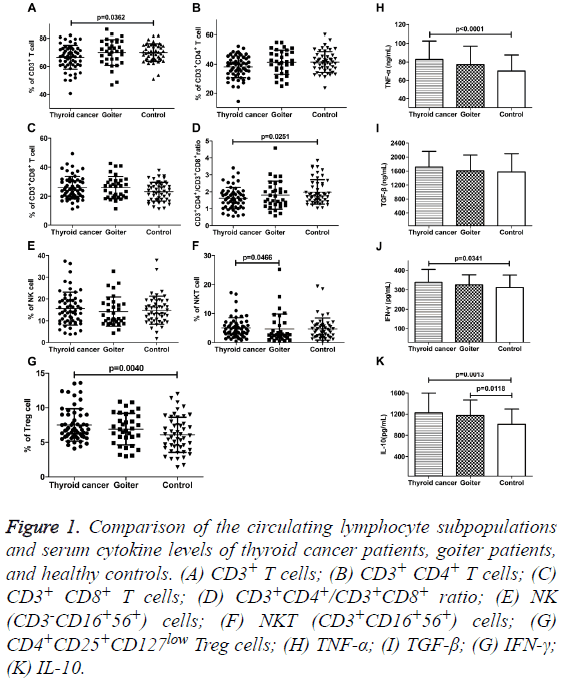
We analyzed the percentages of lymphocyte subsets in peripheral blood of patients with thyroid disease and healthy control by flow cytometry (Figures 1A-1D). The percentages of CD3+ T cells and CD3+CD4+/CD3+CD8+ ratio were both significantly lower in PTC patients than that in control group (66.64 ± 8.55% vs. 69.95 ± 6.64%, p=0.0362; 1.63 ± 0.64 vs. 1.99 ± 0.72, p=0.0251). There were no differences in the proportion of CD3+ T cells and CD3+CD4+/CD3+CD8+ ratio in goiter patients compared with thyroid cancer patients and control. The fractions of CD3+CD4+ and CD3+CD8+ T cells also showed no distinct alteration among the three groups. Although the percentages of CD3+CD8+ T cells in patients with thyroid disease were inclined to rise. The percentages of NK cells (CD3-CD16+56+) in peripheral blood of patients with thyroid disease had no obvious change. However, the proportion of NKT cells (CD3+CD16+56+) in PTC patients was higher than that in goiter patients (5.06 ± 3.50% vs. 4.61 ± 5.19%, p=0.0466), but there was no comparable variance as compared with the control group (Figures 1E and 1F). The populations of CD4+CD25+CD127low Treg among CD4+ T cells were significantly higher (7.52 ± 2.34%) in PTC patients than that in control group (6.10 ± 2.55%, P=0.0040). For goiter patients, there was no obvious change in the percentage of Treg cells while compared with the other two groups (Figure 1G).
Figure 1: Comparison of the circulating lymphocyte subpopulations and serum cytokine levels of thyroid cancer patients, goiter patients, and healthy controls. (A) CD3+ T cells; (B) CD3+ CD4+ T cells; (C) CD3+ CD8+ T cells; (D) CD3+CD4+/CD3+CD8+ ratio; (E) NK (CD3-CD16+56+) cells; (F) NKT (CD3+CD16+56+) cells; (G) CD4+CD25+CD127low Treg cells; (H) TNF-α; (I) TGF-β; (G) IFN-γ; (K) IL-10.
Clinical relevance of CD3+CD4+/CD3+CD8+ ratio and CD4+CD25+CD127low Treg in peripheral blood of patients with thyroid cancer
We next analyzed clinical relevance of CD3+CD4+/CD3+CD8+ ratio and CD4+CD25+CD127low Treg in peripheral blood of PTC patients. The results were presented in Table 2. CD3+CD4+/CD3+CD8+ ratio and the proportion of CD4+CD25+CD127low Treg did not directly correlate with clinicopathologic features in peripheral blood of thyroid cancer patients.
| Characteristics | n | Treg cells (%) | P value | Ratio | P value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 10 | 7.35 ± 2.01 | 0.951 | 1.31 ± 0.60 | 0.124 |
| Female | 49 | 7.35 ± 2.17 | 1.69 ± 0.62 | ||
| Age (y) | |||||
| ≥ 45 | 32 | 7.58 ± 2.36 | 0.543 | 1.78 ± 0.67 | 0.075 |
| <45 | 27 | 7.09 ± 1.84 | 1.46 ± 0.55 | ||
| TNM Stage | |||||
| I+II | 43 | 7.26 ± 1.98 | 0.806 | 1.60 ± 0.63 | 0.533 |
| III+IV | 16 | 7.61 ± 2.56 | 1.72 ± 0.64 | ||
| Metastasis | |||||
| Yes | 20 | 7.40 ± 2.33 | 0.923 | 1.73 ± 0.74 | 0.55 |
| No | 39 | 7.32 ± 2.05 | 1.57 ± 0.57 | ||
Table 2: Correlation between clinicopathologic features and percentage of CD4+CD25+CD127low Treg cells and CD3+CD4+/CD3+CD8+ ratio of PTC patients (mean ± SD).
Increased serum IFN-γ, TNF-α and IL-10 levels in patients with thyroid cancer
Serum IFN-γ, TNF-α, and IL-10 levels were significantly elevated in PTC patients as compared to healthy individuals, IFN-γ: 338.97 ± 65.96 pg/ml vs. 312.00 ± 64.09 ng/ml, P=0.0341; TNF-α: 82.68 ± 20.00 ng/ml vs. 70.10 ± 17.44 ng/ml, P<0.0001; IL-10: 1124.31 ± 374.21 pg/ml vs. 1009.28 ± 288.08 pg/ml, p=0.0013. Meanwhile, only serum IL-10 level was obviously increased in goiter patients (1176.30 ± 290.93 pg/ml vs. 1009.28 ± 288.08 pg/ml, p=0.0118). TGF-β and IL-10 are known to be related to the immunosuppressive function of Treg cells. Our results showed that the levels of serum TGF-β in patients with thyroid carcinoma, goiter and healthy control group were 1171.46 ± 448.46 ng/ml, 1605.52 ± 456.23 ng/ml, and 1573.20 ± 525.53 ng/ml, respectively. There was no significant difference among the three groups (Figures 1H-1K).
In order to confirm the negative results were true negative or due to lack of power, we performed the power calculation using G*Power 3.1.9.2 [9]. The results of Post hoc: Compute achieved power of the above markers showed that, among the three groups, Power (1-β err prob) of CD3+CD4+/CD3+CD8+ ratio, Treg cells, TNF-α, and IL-10 were 0.7495, 0.8050, 0.8342, and 0.7801, respectively. Power of all other markers was less than 0.75.
ROC analysis
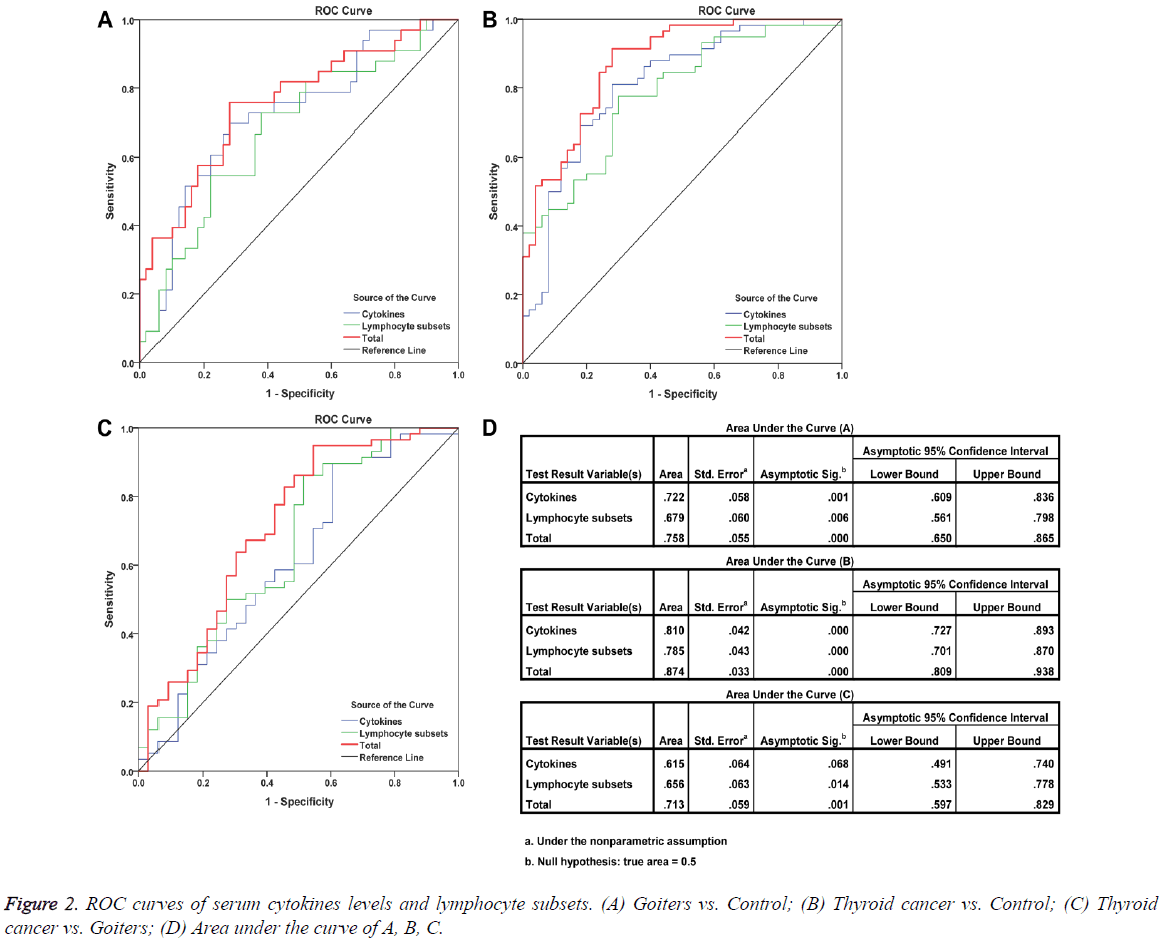
Detection of the percentages of peripheral blood lymphocyte subsets such as CD3+ T cells, CD3+CD4+/CD3+CD8+ ratio, and other markers is an assistant means of clinical examination, which was used to help clinicians determine the immune status of patients. But such examination is not available for cancer diagnosis. To improve the classification efficacy among thyroid cancer, goiters, and controls, we performed the ROC analysis based on lymphocyte subsets, cytokines, and both of them. The results showed that the inclusion of cytokines improved the classification efficacy (Figure 2).
Clinical relevance of serum IFN-γ, TNF-α, IL-10, and TGF-β levels in patients with thyroid cancer
As shown in Table 3, serum IFN-γ, TNF-α, IL-10, and TGF-β levels did not directly correlate with clinicopathologic features in thyroid cancer patients, and there was also no association between the population of Tregs and serum IL-10 and TGF-β levels in these patients (Treg vs. TGF-β: r=0.099, P=0.460; Treg vs. IL-10: r=0.205, P=0.123).
| Characteristics | n | TNF-α | P value | TGF-β | P value | IL-10 | P value | IFN-γ | P value |
|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||
| Male | 10 | 80.01 ± 14.10 | 0.807 | 1752.25 ± 372.71 | 0.699 | 1064.62 ± 217.24 | 0.164 | 347.03 ± 56.22 | 0.669 |
| Female | 49 | 83.24 ± 21.10 | 1702.96 ± 465.70 | 1257.58 ± 392.73 | 337.29 ± 68.23 | ||||
| Age (y) | |||||||||
| ≥ 45 | 32 | 83.66 ± 24.19 | 0.835 | 1680.69 ± 489.26 | 0.4 | 1174.80 ± 338.30 | 0.366 | 337.22 ± 61.08 | 0.994 |
| <45 | 27 | 81.56 ± 14.11 | 1746.78 ± 402.85 | 1281.16 ± 410.69 | 340.97 ± 72.29 | ||||
| TNM stage | |||||||||
| I+II | 43 | 84.16 ± 21.99 | 0.424 | 1682.47 ± 436.95 | 0.347 | 1190.52 ± 314.38 | 0.605 | 343.77 ± 70.06 | 0.267 |
| III+IV | 16 | 78.45 ± 12.32 | 1794.55 ± 485.88 | 1321.19 ± 509.98 | 325.20 ± 52.13 | ||||
| Metastasis | |||||||||
| Yes | 20 | 83.97 ± 26.64 | 0.639 | 1723.68 ± 534.74 | 0.961 | 1302.68 ± 454.24 | 0.307 | 336.60 ± 63.61 | 0.716 |
| No | 39 | 82.00 ± 15.82 | 1705.03 ± 403.57 | 1183.07 ± 323.59 | 340.21 ± 67.97 | ||||
Table 3: Correlation between clinicopathologic features and serum IFN-γ, TNF-α, IL-10, and TGF-β levels (mean ± SD) in PTC patients.
Discussion
The immune system can recognize aggressive tumor cells and maintain the homeostasis by eliminating or inhibiting cancer cells. If the state of immune dysfunction of the host sustained in a certain period, cancer cells may have the opportunity to escape immune surveillance, and then gradually grow into tumor mass. The mechanism by which tumor cells escape immune surveillance is primarily involved in dysfunction of immunosuppressive cells such as Tregs and/or immunomodulatory cytokines. In the past few decades, the incidence of thyroid cancer is increasing, and its development is closely related to the body’s immune dysfunction. Thus, increasing awareness of the changes of immune function involved in the development of thyroid cancer could help clinicians to make more precise personalized therapy. In addition, an important advance in the field of thyroid cancer research was that the infiltration of immune cells could predict prognosis. It might be more important than immune therapy [10,11].
As we know, compared with the assessment of immune cells infiltration, detection of peripheral blood lymphocyte subsets in thyroid cancer patients is a relatively less effective method, but for the newly diagnosed patients, it is a moderately more convenient and effective means, which could assess the immune function of patients, and assist in clinical diagnosis and treatment. In particular, with the development of multicolor flow cytometry and other technologies, we can simultaneously detect more parameters, so as to reduce the burden on patients and to improve the efficiency [4,12].
CD8+ and CD4+ T cells play a synergistic role in immune responses. CD8+ T cells, which are generally involved in the adaptive immune response, are primarily responsible for the elimination of tumor cells. CD4+ T cells also play a central role in immune response. Naïve CD4+ T cells can differentiate into four populations: Th1, Th2, Tregs, and Th17 cells. Th1 cells promote CD8+ T cell-mediated cytotoxicity and secrete IL-2, IFN-γ, and other cytokines, which associated with inflammation and cell-mediated immune response. Th2 cells stimulate humoral immune response and produce IL-4, IL-5, IL-10 and others. The role of Tregs is to inhibit the immune response by releasing IL-10 and TGF-β. In this study, our results showed that there was no statistical difference in the percentages of CD3+ CD4+ and CD3+ CD8+ T cells in peripheral blood among thyroid cancer patients, goiters and healthy controls. But the proportion of CD3+ T cells and CD3+ CD4+/CD3+ CD8+ ratio in PTC patients was significantly lower than that in the control group, indicating that the immune function of these patients was impaired. However, the immune status of thyroid cancer patients was not associated with sex, age, TNM stage, and metastasis.
Natural killer cells are effectors of innate immune response that can recognize and eliminate pathogens and produce a variety of cytokines such as TNF-α, IFN-γ, and Granulocyte- Macrophage Colony-Stimulating Factor (GM-CSF), and influence the function of dendritic cells, macrophages and neutrophils through their cytotoxicity and cytokine production. Therefore, it is more accurate to say that NK cells have a regulatory function, which can affect the immune response of the antigen specific T cells and B cells. Gogali et al. evaluated the degree of NK cells’ infiltration in 65 patients with PTC and 25 patients with nodular goiter. They found an increased number of NK cells infiltration in the tumor tissue samples, and there was a negative correlation between the degree of NK cell infiltration and the clinical stage of cancer patients. They also found that the distribution of immunoregulatory and cytotoxic NK cells in peripheral blood of patients with PTC, nodular goiter and healthy controls were similar, but the number of immunoregulatory NK cells in the tumor microenvironment of PTC patients increased. Consistent with previous reports, in this study, we compared the changes in the number of NK (CD3-CD16+56+) cells in the peripheral blood of patients with thyroid disease and healthy controls. The results showed that there was no significant difference among the percentages of NK cells in thyroid cancer patients, goiter patients and controls [13,14]. There is a certain degree of consistency between the results of our experiments and other previous reports, and of course there are some differences, which are mainly due to the variances in the selected clinical subjects. Our study focused on newly diagnosed thyroid cancer patients, while others might include patients receiving chemotherapy and radiotherapy [15,16].
Regulatory T cells (Tregs) play an essential role in maintaining homeostasis and protecting the host against autoimmune diseases. Tregs can inhibit the autoimmune attack and antitumor immunity mediated by T cells, and can induce tolerance through inhibiting the effects of CD4+ T cells, CD8+ T cells, NK cells, dendritic cells, mast cells, monocytes/ macrophages. Treg cells also play an important role in tumor immunological escape by secreting inhibitory cytokines such as TGF-β and IL-10. The increasing number of Tregs in tumor patients is closely related to disease progression and poor prognosis. Transcription factor fork head box p3 (Foxp3) plays a key part in the development and function of Treg cells. Muller et al. observed that the number of Foxp3+ Treg cells in peripheral blood of medullary thyroid carcinoma patients was higher than that in patients with benign goiter, and was associated with prognosis [17]. In addition, the low expression of CD127 (the alpha chain of the IL-7 receptor) and high expression of CD25 can better identify and purify Treg cells with prominent inhibitory effect. Therefore, CD4+CD25+CD127low could serve as markers for Tregs. We examined the percentage of CD4+ CD25+ CD127low Treg cells in peripheral blood of patients with thyroid cancer and goiter, and found that the proportion of Tregs in thyroid cancer patients was higher than that in healthy controls. This result can be explained by the fact that Treg cells can reduce tumorspecific immune-mediated inflammation, which can in turn drive the development of the tumor. We then analyzed the correlation between the population of Treg cells and the clinicopathological features of thyroid cancer patients. The results showed that the percentage of Treg cells in these patients was not directly related to the sex, age, TNM staging and metastasis. This result is different from other reports. In our study, the number of patients with early stage thyroid cancer was higher than that of advanced patients. Therefore, although the number of Tregs in advanced patients was higher than that in early stage patients, there was no statistical variation between them. We will increase the number of patients enrolled in subsequent study [18-20].
Cytokines are key mediators of inflammation, and some special cytokines are also hallmarks of cancer. Thyroid disease is closely related to inflammation and often manifested as inflammatory disorders, which may be reflected by the patient’s serum cytokine profile. Therefore, in the process of diagnosis and treatment of thyroid cancer, the detection of serum cytokine profiles is a potential means to promote the development of diagnosis and management of thyroid cancer [4]. In this study, we detected 4 kinds of serum cytokines including IFN-γ, TNF-α, IL-10, and TGF-β [21,22].
TNF-α is a 17 kDa cytokine, and is primarily produced by activated macrophages, T lymphocytes, and NK cells. It is an important mediator of inflammation and cancer. TNF-α seems to be having complicated roles in cancer, not only can induce tumor necrosis, but also promote tumor growth, invasion and metastasis [6]. Within cells, TNF-α activates different signalling pathways by binding to its receptors, thereby regulating cell survival, proliferation, and death. It can also activate endothelial cells, causing them to produce adhesion molecules that attract neutrophils, monocytes and lymphocytes. One study enrolled 150 untreated patients with 67 benign thyroid diseases, 83 PTC, and 67 healthy individuals to detect the circulating levels of TNF-α, L-Selectin, and VCAM-1, and found that serum levels of TNF-α, L-Selectin, and VCAM-1 were significantly higher in patients with both benign thyroid diseases and PTC as compared to the healthy individuals. The results signified the probable role of TNF-α and the adhesion molecules in thyroid carcinogenesis, suggested that the circulating TNF-α levels could be used as a prognosticator for OS in PTC patients [5]. Similar results were obtained from our study. But Linkov et al. observed that patients with thyroid disease (23 thyroid cancer and 24 benign thyroid disease) tended to have lower TNF-α than the reference normal (N=23), although the difference was not statistically significant [23]. Simonovic et al. also found that TNF-α level in peripheral blood of differentiated thyroid cancer patients did not change apparently [24].
IFN-γ can directly affect the immunogenicity and promote the recognition and elimination of tumor cells. It can destruct autoimmune thyrocyte through the up-regulation of CD95, caspase-3, and caspase-8 expression [25]. Chronic low levels of TNF-α and IFN-γ were found to enhance the capacity of PTC cell lines to migrate and invade through the SMAD, NF- κB, AKT/GSK-3β, and JAK/STAT signalling pathways, and this process coincided with the down regulation of E-cadherin and up regulation of N-cadherin and vimentin, which are hallmarks of Epithelial-Mesenchymal Transition (EMT) [6,26]. Kobawala et al. determined serum IL-8 and IFN-α from a total of 88 individuals of which 19 were healthy individuals, and 69 were patients with thyroid diseases: goitre (N=21), autoimmune diseases (N=16), and carcinomas (N=32). Both IL-8 and IFN-α were significantly higher in all the patients as compared to healthy individuals. Serum IL-8 levels showed significant positive correlation with disease stage in thyroid cancer patients. While no significant correlation was observed between serum IFN-α levels and any of the clinicopathological parameters [27].
Th2-type cytokines, such as TGF-β and IL-10, secreted by thyroid cancer cells, are mainly involved in immunosuppression. TGF-β is a potent immunosuppressive factor, which can inhibit the proliferation of thyroid cells and the secretion of immunoglobulin (IgG) M, IgG1, IgG2a, and IgG3, and stimulate the occurrence of EMT. IL-10 promotes the expression of anti-apoptotic protein Bcl-2 and Bcl-xL, and protects tumor cells against the attack of chemotherapeutic drugs. It can also lead to constitutive activation of the JAK/ STAT pathway, and up-regulate the expression of cFLIPL and PED, so that make cancer patients unable to respond to the stimulation of CD95. Elevated IL-10 has been shown to cause immunosuppression in patients with a variety of cancers including melanoma, head and neck tumors, pancreatic, gastric, lung, and breast cancer, by down-regulating the expression of MHC class I molecules or inhibiting the activation of T cells [7,28-31]. Vesely et al. measured the concentrations of IGF-I, HGF, TGFβ1, bFGF, and VEGF in the serum of 28 patients with thyroid gland tumors (14 adenomas, 14 PTC) and 8 healthy people. They found significantly lower serum levels of IGF-I in patients with thyroid adenoma compared to the healthy population. Serum levels of HGF and bFGF were significantly higher in patients with thyroid adenoma and papillary carcinoma compared with those in healthy subjects. Serum concentrations of TGFβ1 and VEGF were not significantly different in any groups of investigated subjects. Our results consistently indicated that there was no significant difference in serum TGF-β level among patients with thyroid cancer, goiters and healthy controls [32].
In recent years, it was demonstrated that malignant and benign thyroid conditions are associated with altered expression levels of interleukins. In particular, significantly higher levels of IL-6, IL-7, IL-10, and IL-13, as well as significantly lower levels of IL-8, were observed in patients with benign and malignant thyroid cancer compared to controls [29]. In our study, the levels of serum TNF-α, IFN-γ, and IL-10 in thyroid cancer patients were higher than those in healthy controls, but there was no variance between thyroid cancer patients and goiters.
Dissimilarities in results of the reported studies may be related to the difference in the number of patients studied and in their distinct pathological characteristics. In order to confirm the negative results were true negative or due to lack of power, we performed the power calculation using G*Power 3.1.9.2 [9]. The results showed that CD3+CD4+/CD3+CD8+ ratio, Treg cells, TNF-α, and IL-10 had relatively stronger test efficiency. Further analyse indicates that the levels of serum IFN-γ, TNF- α, IL-10, and TGF-β in patients with thyroid cancer did not show any correlation with clinicopathological features. However, ROC curve analysis suggested that TNF-α, TGF-β, IL-10, and IFN-γ exhibited certain discriminant effects between healthy controls and thyroid cancer patients (TNF-α: AUC=0.743, P=0.000; TGF-β: AUC=0.617, P=0.036; IL-10: AUC=0.662, P=0.004; IFN-γ: AUC=0.607, P=0.057), but they could not discriminate patients with benign thyroid diseases from thyroid cancer patients. Among the detected four cytokines, only the level of serum IL-10 in goiter patients was higher than that in healthy controls. ROC curves also validated that IL-10 was a potential marker for distinguishing patients with goiters from the healthy controls (AUC=0.683, P=0.005). We next performed a multiple factor set ROC curve analysis for the four cytokines and lymphocyte subsets. The results showed that combined detection of cytokines and lymphocyte subsets exhibited a good ability to distinguish thyroid cancer patients, goiters, and healthy individuals. This distinguishing ability was more powerful than those using cytokines or lymphocyte subsets alone.
There are some limitations in our study. The prominent problem is the sampling method. The retrospective basis of our evaluation and lack of randomization engendered selection bias. We would correct this shortcoming in our on-going work by using propensity score matching. Certainly, we need to expand the sample size as much as possible. In this study, we selected some general markers of lymphocyte subsets and cytokines to monitor the immune function of thyroid cancer patients. The existed results motivate us to do more intensive work. At present, there are some approaches that can simultaneously detect multiple markers, such BD CBA (Cytometric Bead Array). These measures can greatly improve the detection efficiency and accuracy. Therefore, additional studies are required to widen our understanding to the complicated immune networks in cancer patients for the identification of new targets that could lead to improved diagnosis and treatment.
Conclusion
In conclusion, the results of this study showed that the immune functions of thyroid cancer patients were impaired, which were mainly reflected in their higher proportion of Treg cells, but the complex role of cytokines still needs in-depth study. Combined detection of cytokines and lymphocyte subsets exhibited a good ability to distinguish thyroid cancer patients, goiters, and healthy individuals. These observations stimulated us to focus on predicting prognosis and assessing clinical outcomes of patients in the future.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation (Jilin Cancer Hospital) and with the Helsinki Declaration of 1975, as revised in 2008.
Competing Interests
None declared.
References
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. Cancer J Clin 2016; 66: 115-132.
- Imam S, Paparodis R, Sharma D, Jaume JC. Lymphocytic profiling in thyroid cancer provides clues for failure of tumor immunity. Endocr Relat Cancer 2014; 21: 505-516.
- Cunha LL, Morari EC, Guihen AC, Razolli D, Gerhard R, Nonogaki S, Soares FA, Vassallo J, Ward LS. Infiltration of a mixture of immune cells may be related to good prognosis in patients with differentiated thyroid carcinoma. Clin Endocrinol 2012; 77: 918-925.
- Capone F, Guerriero E. Serum cytokinome profile evaluation: A tool to define new diagnostic and prognostic markers of cancer using multiplexed bead-based immunoassays. Mediators Inflamm 2016; 2016: 3064643.
- Fugazzola L, Colombo C, Perrino M, Muzza M. Papillary thyroid carcinoma and inflammation. Front Endocrinol 2011; 2.
- Kobawala TP, Trivedi TI, Gajjar KK, Patel DH, Patel GH, Ghosh NR. Significance of TNF-alpha and the Adhesion Molecules: L-Selectin and VCAM-1 in Papillary Thyroid Carcinoma. J Thyroid Res 2016; 2016: 8143695.
- Stanciu AE, Serdarevic N, Hurduc AE, Stanciu MM. IL-4, IL-10 and high sensitivity-CRP as potential serum biomarkers of persistent/recurrent disease in papillary thyroid carcinoma with/without Hashimoto's thyroiditis. Scand J Clin Lab Invest 2015; 75: 539-548.
- Jiang L, Zhan Y, Gu Y, Ye Y, Cheng Y, Shi H. Changes of regulatory T and B cells in patients with papillary thyroid carcinoma after 131I radioablation: a preliminary study. BioMed Res Int 2013; 2013: 683768.
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175-191.
- Nishant A, Rehan A, Arman A, Adrian A. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014; 159: 676-690.
- Piccardo A, Arecco F, Puntoni M, Foppiani L, Cabria M, Corvisieri S, Arlandini A, Altrinetti V, Bandelloni R, Orlandi F. Focus on high-risk DTC patients: high postoperative serum thyroglobulin level is a strong predictor of disease persistence and is associated to progression-free survival and overall survival. Clin Nucl Med 2013; 38: 18-24.
- Modi J, Patel A, Terrell R, Tuttle RM, Francis GL. Papillary thyroid carcinomas from young adults and children contain a mixture of lymphocytes. J Clin Endocrinol Metabol 2003; 88: 4418-4425.
- Gogali F, Paterakis G, Rassidakis GZ, Kaltsas G, Liakou CI, Gousis P, Neonakis E, Manoussakis MN, Liapi C. Phenotypical analysis of lymphocytes with suppressive and regulatory properties (Tregs) and NK cells in the papillary carcinoma of thyroid. J Clin Endocrinol Metabol 2012; 97: 1474-1482.
- Gogali F, Paterakis G, Rassidakis GZ, Liakou CI, Liapi C. CD3 (-) CD16 (-) CD56 (bright) immunoregulatory NK cells are increased in the tumor microenvironment and inversely correlate with advanced stages in patients with papillary thyroid cancer. Thyroid 2013; 23: 1561-1568.
- Weber F. Lymphocytes and thyroid cancer: more to it than meets the eye? Endocr Relat Cancer 2014; 21: 1-5.
- Severson JJ, Serracino HS, Mateescu V, Raeburn CD, McIntyre RC Jr, Sams SB, Haugen BR, French JD. PD-1+Tim-3+ CD8+ T lymphocytes display varied degrees of functional exhaustion in patients with regionally metastatic differentiated thyroid cancer. Cancer Immunol Res 2015; 3: 620-630.
- Muller S, Poehnert D, Muller JA, Scheumann GW, Koch M, Luck R. Regulatory T cells in peripheral blood, lymph node, and thyroid tissue in patients with medullary thyroid carcinoma. World J Surg 2010; 34: 1481-1487.
- Lim KP, Chun NA, Ismail SM, Abraham MT, Yusoff MN, Zain RB, Ngeow WC, Ponniah S, Cheong SC. CD4+CD25hiCD127 low regulatory T cells are increased in oral squamous cell carcinoma patients. PloS One 2014; 9: e103975.
- French JD, Weber ZJ, Fretwell DL, Said S, Klopper JP, Haugen BR. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J Clin Endocrinol Metabol 2010; 95: 2325-2333.
- Suzuki S, Shibata M, Gonda K, Kanke Y, Ashizawa M, Ujiie D, Suzushino S, Nakano K, Fukushima T, Sakurai K, Tomita R, Kumamoto K, Takenoshita S. Immunosuppression involving increased myeloid-derived suppressor cell levels, systemic inflammation and hypoalbuminemia are present in patients with anaplastic thyroid cancer. Mol Clin Oncol 2013; 1: 959-964.
- Liotti F, Visciano C, Melillo RM. Inflammation in thyroid oncogenesis. Am J Cancer Res 2012; 2: 286-297.
- Zivancevic-Simonovic S, Mihaljevic O, Majstorovic I, Popovic S, Markovic S, Milosevic-Djordjevic O, Jovanovic Z, Mijatovic-Teodorovic L, Mihajlovic D, Colic M. Cytokine production in patients with papillary thyroid cancer and associated autoimmune Hashimoto thyroiditis. Cancer Immunol Immunotherap 2015; 64: 1011-1019.
- Linkov F, Ferris RL, Yurkovetsky Z, Marrangoni A, Velikokhatnaya L, Gooding W, Nolan B, Winans M, Siegel ER, Lokshin A, Stack BC Jr. Multiplex analysis of cytokines as biomarkers that differentiate benign and malignant thyroid diseases. Proteom Clin Appl 2008; 2: 1575-1585.
- Simonovic SZ, Mihaljevic O, Majstorovic I, Djurdjevic P, Kostic I, Djordjevic OM, Teodorovic LM. Cytokine production in peripheral blood cells of patients with differentiated thyroid cancer: elevated Th2/Th9 cytokine production before and reduced Th2 cytokine production after radioactive iodine therapy. Cancer Immunol Immunotherap 2015; 64: 75-82.
- Stassi G, Di Liberto D, Todaro M, Zeuner A, Ricci-Vitiani L, Stoppacciaro A, Ruco L, Farina F, Zummo G, De Maria R. Control of target cell survival in thyroid autoimmunity by T helper cytokines via regulation of apoptotic proteins. Nat Immunol 2000; 1: 483-488.
- Lv N, Gao Y, Guan H, Wu D, Ding S, Teng W, Shan Z. Inflammatory mediators, tumor necrosis factor-alpha and interferon-gamma, induce EMT in human PTC cell lines. Oncol Lett 2015; 10: 2591-2597.
- Kobawala TP, Patel GH, Gajjar DR, Patel KN, Thakor PB, Parekh UB, Patel KM, Shukla SN, Shah PM. Clinical utility of serum interleukin-8 and interferon-alpha in thyroid diseases. J Thyroid Res 2011; 2011: 270149.
- Zivancevic-Simonovic S, Mihaljevic O, Mihajlovic D, Milosevic-Djordjevic O, Jovanovic Z, Mijatovic-Teodorovic L, Colic M. Transforming growth factor beta 1 (TGF-beta1) in thyroid cancer patients: a view from the peripheral blood. Ann Clin Lab Sci 2016; 46: 401-406.
- Provatopoulou X, Georgiadou D, Sergentanis TN, Kalogera E, Spyridakis J, Gounaris A, Zografos GN. Interleukins as markers of inflammation in malignant and benign thyroid disease. Inflamm Res 2014; 63: 667-674.
- Todaro M, Zerilli M, Ricci-Vitiani L, Bini M, Perez Alea M, Maria Florena A, Miceli L, Condorelli G, Bonventre S, Di Gesu G, De Maria R, Stassi G. Autocrine production of interleukin-4 and interleukin-10 is required for survival and growth of thyroid cancer cells. Cancer Res 2006; 66: 1491-1499.
- Drennan S, Stafford ND, Greenman J, Green VL. Increased frequency and suppressive activity of CD127 (low/-) regulatory T cells in the peripheral circulation of patients with head and neck squamous cell carcinoma are associated with advanced stage and nodal involvement. Immunol 2013; 140: 335-343.
- Vesely D, Astl J, Lastuvka P, Matucha P, Sterzl I, Betka J. Serum levels of IGF-I, HGF, TGFbeta1, bFGF and VEGF in thyroid gland tumors. Physiol Res 2004; 53: 83-89.