ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 20
High resistance rates to 2nd and 3rd generation cephalosporins, ciprofloxacin and gentamicin of the uropathogens isolated in young infants hospitalized with first urinary tract infection
Oana Falup-Pecurariu1,2*, Eugene Leibovitz3, Mihaela Bucur1, Raluca Lixandru1, Laura Bleotu1, Cristian Falup-Pecurariu2
1Children’s Clinic Hospital, Bra?ov, România
2Faculty of Medicine, Transilvania University, Bra?ov, România
3Pediatric Infectious Disease Unit, Soroka University Medical Center, Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel
- *Corresponding Author:
- Oana Falup-Pecurariu
Children’s Clinic Hospital, Bra?ov, România Faculty of Medicine Transilvania University România
Accepted date: September 27, 2017
Aims: To describe the characteristics of febrile UTI in hospitalized infants and to characterize the uropathogens distribution and antimicrobial resistance rates.
Methods: Retrospective study performed during 2010-2013, including all infants <3 months of age admitted with the diagnosis of UTI proven by urine culture obtained by bladder catheterization.
Results: 117 infants with 1st UTI episode were enrolled. There were 18 (15.39%), 57 (48.72%) and 42 (35.9%) infants aged 0-1, 1-2 and 2-3 months; 5.99% had previous renal anomalies. Fever >38°C at admission was recorded in 33 (28.2%). Leukocytosis, leukopenia and neutropenia were recorded in 20 (17.1%), 2 (1.7%) and 5 (4.3%) patients, respectively. Escherichia coli, Klebsiella spp., Enterococcus spp., Morganella morganii, Proteus spp. and Enterobacter spp. were the most common pathogens (53.3%, 10.6%, 5.2%, 5.2%, 4.5%, and 3.9% of all episodes, respectively). No differences were recorded between E. coli or Klebsiella spp.-UTI cases recorded in male vs. female patients and between the 3 age subgroups. The antibiotic resistance rates of E. coli were 61.8%, 57.4%, 50% and 45.6% for ceftriaxone, cefuroxime, gentamicin and ciprofloxacin, respectively. The antibiotic resistance rates of Klebsiella spp. were 82.9%, 80%, 54.3% and 54.3% for ceftriaxone, cefuroxime, gentamicin and ciprofloxacin, respectively. 80.9% and 42.9% of the E. coli and Klebsiella spp. isolates were ESBL-producers. The resistance rates of the 2 major pathogens to piperacillin/tazobactam, meropenem, nalidixic acid, chloramphenicol and colistin were low.
Conclusion: The high resistance rates to major antibiotic classes of uropathogens isolated in hospitalized infants with UTI require close periodically monitoring and may require modification of empirical antibiotic therapies in use for these patients.
Keywords
Infants, Urinary tract infection, E. coli, Klebsiella spp., Antibiotics, Nonsusceptibility.
Introduction
Urinary tract infection (UTI) represents one of the most common bacterial infections in children and one of the main reasons for fever and antibiotic prescriptions. The incidence of the disease reaches 3% in neonates and is around 0.7% in infants up to 1 year of age [1-4]. The prevalence of UTI in febrile infants is around 5% [5-7]. Gram-negative bacteria represent the most common etiologic cause of this condition and, among them, the most frequently isolated is Escherichia coli, causing up to 70-90% of all UTIs [1-4]. Other causative bacteria are Proteus spp. (mostly commonly encountered in boys <1 year of life), Enterobacter spp. and Klebsiella spp. Of the Gram-positive bacteria, Enterococcus fecalis is the most commonly isolated pathogen. UTI must be taken into consideration when young infants are examined for fever because it could be a serious condition with almost one-third of cases having associated bacteremia [1-4].
Diagnosis of UTI is of major importance, particularly in children, because this infection may represent, potentially, the first and only sign of a congenital defect of the urinary tract. Early diagnosis and treatment will prevent complications such as renal scarring, deteriorating renal function and hypertension, especially in young infants and children <5 years of age [4-7]. Approximately 30% to 50% of young infants with UTI may have urinary tract abnormalities, of which vesicoureteral reflux (VUR) is the most common. The clinical diagnostic and treatment approach in UTI were summarized in clinical guidelines published by the American Academy of Pediatrics and several other national forums with minor changes [8-11]. The last guidelines published in 2011 consider that performance of voiding cystourethrography (VCUG) is not recommended anymore in the investigation of a first UTI episode and that early antimicrobial treatment may decrease the risk of renal damage for UTI [9-11].
Diagnosis is made on the basis of the presence of both pyuria and at least 50 000 colonies/ml of a single uropathogenic organism in an appropriately collected specimen of urine [9-11]. After 7-14 days of antimicrobial treatment, close clinical follow-up monitoring should be maintained to permit prompt diagnosis and treatment of recurrent infections. Ultrasonography of the kidneys and bladder should be performed to detect anatomic abnormalities. Today, the need for all or part of blood and imaging investigations is controversial, especially in infants who already had their urinary tract investigated by US during pregnancy [9-11].
To ensure appropriate treatment, knowledge of the pathogens causing UTI and their antibiotic susceptibility is mandatory. In recent years, an increasing proportion of uropathogens are resistant to antibiotics, with increasing numbers of failures in treatment after empiric therapy recommended by national and international guidelines for children [12,13].
We performed a retrospective study whose specific purposes were to describe the characteristics of first UTI episodes diagnosed in 0-3-month-old infants as part of a systematic screening for UTI in children seen in the emergency department and subsequently hospitalized, to characterize the distribution of uropathogens and antimicrobial resistance rates as function of patient age and gender and to evaluate the antibiotic treatment policy at our medical center.
Materials and Methods
This retrospective study was performed during 2010-2013 and included all the infants <3 months of age admitted to the pediatric department 1A of the Children’s Hospital (which admits all non-surgical infants <3 months of age hospitalized in the hospital) with the diagnosis of UTI proven by urine culture. Our hospital is the only primary and tertiary medical center in the city of Brasov (located in Central Romania) and takes care of a population of approximately 300,000 patients (of them around 100,000 children).
Diagnosis of UTI was made on the basis of the presence of at least 50.000 colonies/ml of a single uropathogenic organism in a specimen of urine obtained by bladder catheterization. The medical records of the admitted infants, laboratory findings from the bacteriological laboratory and imaging data from the radiology department were searched. Age, sex and ethnicity of the infants with UTI were documented. The departmental protocol for the initial empiric treatment of infants with suspicion of UTI included 1) ampicillin (100 mg/kg/day three times/day plus gentamicin (5 mg/kg/day once/day) or 2) cefuroxime (100 mg/kg/day three times/day) or 3) ceftriaxone 50 mg/kg/day once/day). The intravenous antibiotic treatment was continued for a minimum of 7 days and changed to an oral antibiotic (cephalexin 50 mg/kg three times/day in most cases) for 3-7 additional days, summarizing a total of 10-14 days of antibiotic therapy. Anatomical abnormalities diagnosed during the first episode of UTI were documented as well as all imaging finding completed during the hospitalization. The departmental policy required the performance of a follow-up urine culture at discharge. The antibiotic prophylaxis policy was not well defined at the pediatric department during the study period, leaving at the physicians' decision the specific antibiotic to be administered. However, the local guidelines recommended antibiotic prophylaxis for all infants <3 months of age with first UTI episodes until the completion of imaging studies. The recommended imaging studies during the study period included the completion of an ultrasound (US) examination during hospitalization or after hospitalization in all cases of infants <3 months of age with UTI. Additional investigations, like VCUG and Technetium- Dimercaptosuccinic (DMSA) scan were performed according to nephrologist’s recommendations.
The study was approved by the ethics committee of the hospital.
Statistical analysis: Data were recorded using the Access Microsoft Office software. Statistical analysis was performed using the SPSS 19.0 software. Contingency table analysis for comparing rates between unmatched samples was performed using the Chi-square or Fisher’s exact test, as appropriate. Student’s independent samples t-test or ANOVA were used to compare continuous variables.
Results
Overall, 117 infants <3 month of age diagnosed with the 1st UTI episode in life were enrolled. These infants represented 12.0% of all 975 infants <3 months of age admitted at the pediatric departments during the study years (29/256 [11.3%], 36/239 [15.1%], 23/250 [9.2%], and 29/233 [12.4%]) during 2010, 2011, 2012 and 2013, respectively).
The mean ± standard deviation age (in months) at UTI diagnosis was 1.48 ±0.8 for the whole study population; no differences were recorded in the mean age at first UTI diagnosis between male and female infants (1.46 ± 0.85 vs.1.39 ± 0.84 months, P=0.67). There were 74 (63.2%) male patients (all not circumcised) and 43 (36.8%) female patients. There were 18 (15.4%), 57 (48.7%) and 42 (35.9%) infants aged 0-1, 1-2 and 2-3 months at the age of UTI diagnosis. The mean (±SD) gestational age of the study patients was 38.1 ± 1.97 weeks (median 38, range 32-40 weeks). None of the patients were <30 weeks gestational age; 12 (10.3%) were <36 weeks gestational age. The mean (±SD) birth weight was 2940.34 ± 518.76 g (median 3000, range 1500-4000 g; 6/117 (5.1%) had a birth weight <2000 g. None of the 117 infants received any antibiotic therapy prior to the present hospitalization with UTI.
One hundred and seven (91.5%) young infants with UTI did not suffer from any previous pathologic conditions. The remaining 10 (8.5%) were diagnosed with various anomalies, of them 7 (6.0%) with renal anomalies (5 hydronephrosis, 1 polycystic kidney and 1 congenital megaurether), 1 atrial septal defect, 1 ventricular septal defect and 1 with milk allergy. Concomitant pathologies (conditions) were diagnosed at the time of UTI diagnosis in 69 (59.0%) patients with UTI, of them acute gastroenteritis, bronchiolitis, dehydration and pneumonia in 22 (18.8%), 14 (12.0%), 11 (9.4%) and 6 (5.1%) cases.
Fever>38°C at admission was recorded in 33 (28.2%) (Table 1). None of the infants was hypothermic at admission. Leukocytosis, leukopenia and neutropenia were recorded in 20 (17.1%), 2 (1.7%) and 5 (4.3%) patients, respectively. CRP was determined in 73 (62.4 %) patients and was abnormal in 42.5% of them. Renal ultrasound was performed during hospitalization in all patients and confirmed the previously diagnosed renal pathologies in all 7 cases; an additional patient was found with hydronephrosis.
| Fever at admission | No. patients (%) |
|---|---|
| <38.0°C | 84 (71.8 %) |
| 38.1-39.0°C | 29 (24.8 %) |
| >39.1°C | 4 (3.4 %) |
| WBC/mm3 | |
| Mean ± SD | 12.0 ± 5.1 |
| Range | 2.4-33.500 |
| Leukocytosis>15.000 | 20 (17.1%) |
| Leukopenia <5000 | 2 (1.7%) |
| Polymorphonuclears (mean ± SD) | 4.0 ± 2.9 |
| Neutropenia<1500 | 5 (4.3%) |
| Hb = 10 mg | 20 (17.1%) |
| Platelets (mean ± SD) | 438.1 ± 152.4 |
| >150.000/mm3 | 117 (100%) |
| CRP (mg/dl)* | |
| No. patients with available data=73 | |
| Mean ± SD | 3.6 ± 4.9 |
| Range | 0.19-21.65 |
| >1 mg/dl | 31 (42.5%) |
| Urea>40 mg (%) | 5 (4.3%) |
| Creatinine>0.4 mg (%) | 37 (31.6%) |
| Abnormal liver function tests (%) | 30 (25.6%) |
*Normal value: ≤1mg/dl.
Table 1: Fever and laboratory findings at admission.
Microbiology: One single uropathogen was isolated in each of the 117 UTI episodes. Escherichia coli, Klebsiella spp., Enterococcus spp., Morganella morganii, Proteus spp., and Enterobacter spp. were the most frequently isolated pathogens (53.3%, 10.6%, 5.2%, 5.2%, 4.5%, and 3.9% of all episodes, respectively) (Table 2). Mixed infections (caused by two organisms considered as true UTI pathogens) were not recorded. Overall, there were 45 (38.47%) non-E. coli Gramnegative pathogens. No statistical differences were recorded between E. coli or Klebsiella spp.-UTI cases recorded in male vs. female patients (40, 54.1% vs. 28, 65.1%, P=0.24 and 24, 32.4% vs. 11, 25.6%, P=0.44, respectively).
| Pathogens | n (%) |
|---|---|
| - Escherichia coli | 68 (58.1%) |
| - Klebsiella spp. | 35 (29.9%) |
| - Proteus spp. | 5 (4.3%) |
| - Enterobacter spp. | 3 (2. 6%) |
| - Pseudomonas aeruginosa | 2 (1.7%) |
| - Enterococcus spp. | 2 (1.7%) |
| - Methicillin-Resistant Staphylococcus aureus | 2 (1.7%) |
| - Total | 117 |
Table 2: Pathogen distribution: 117 cases of UTI in 117 infants aged 0-3 months.
No statistical differences were recorded in the distribution of E. coli or Klebsiella spp. between the 3 age subgroups (0-1, 1-2 and 2-3 months of age) (Table 3). No statistical differences were recorded in the distribution of E. coli vs. non-E. coli Gram-negative pathogens between the 3 age subgroups. The blood cultures performed in all patients returned negative. An additional urine culture was performed during therapy in 30 (25.6%) patients and was negative in all of them. A urine culture (by bag) was performed at discharge in all patients and returned negative in all of them.
| Pathogen | 0-1 months (n=44) | 1-2 months (n=43) | 2-3 months (n=30) | Total (N=117) |
|---|---|---|---|---|
| Escherichia coli | 24 (54.5%) | 25 (58.2%) | 19 (63.3%) | 68 |
| Klebsiella spp. | 13 (29.6%) | 14 (32.6%) | 8 (26.7%) | 35 |
| Proteus spp. | 2 (4.5%) | 1 (2.3%) | 2 (6.7%) | 5 |
| Enterobacter spp. | 2 (4.5%) | 1 (2.3%) | - | 3 |
| Enterococcus spp. | 1 (2.3%) | 1 (2.3%) | - | 2 |
| Pseudomonas spp. | 1 (2.3%) | - | 1 (3.3%) | 2 |
| Non-E. coli gram-negative organisms | 19 (43.2%) | 17 (39.5%) | 11 (36.7%) | 47 |
| Methicillin-resistant Staphylococcus aureus | 1 (2.3%) | 1 (2.3%) | - | 2 |
Table 3: Pathogen distribution according to age subgroups.
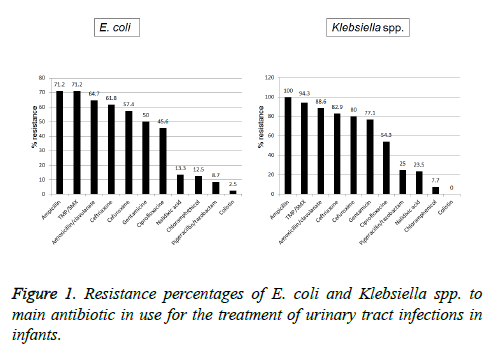
Antibiotic susceptibility (Figure 1): The antibiotic resistance rates of the E. coli isolates tested to the most commonly used antibiotics were 62/28 (71.2%) for ampicillin and TMP/SMX, followed by 44/68 (64.7%), 42/68 (61.8%), 39/68 (57.4%), 34/68 (50%), 31/68 (45.6%), 6/45 (13.3%), 3/24 (12.5%), 2/23 (8.7%) and 1/40 (2.5%) and for amoxicillin/clavulanic acid, ceftriaxone, cefuroxime, gentamicin, ciprofloxacin, nalidixic acid, chloramphenicol, piperacillin/tazobactam and colistin, respectively. The antibiotic resistance of Klebsiella spp. isolates tested was 35/35 (100%), 33/35 (94.3%), 31/35 (88.6%), 29/35 (82.9%), 28/35 (80%), 27/35 (54.3%), 19/35 (54.3%), 3/12 (25%), 4/17 (23.5%), 2/26 (7.7%) and 0/22 (0%) for ampicillin, TMP/SMX, amoxicillin/clavulanic acid, ceftriaxone, cefuroxime, gentamicin, ciprofloxacin, piperacillin/tazobactam, nalidixic acid, chloramphenicol and colistin, respectively.
All 11 pathogens (7 E. coli, 2 Klebsiella spp., 1 Pseudomonas spp. and 1 MRSA) tested were found susceptible to amikacin. Meropenem was tested for 11 patients and 10/11 pathogens isolated (6/6 E. coli and 5/6 Klebsiella spp.) were found susceptible to this antimicrobial.
Fifty-five (80.9%) of the 68 E. coli were ESBL-positive; 15/35 (42.9%) of the Klebsiella spp. tested were ESBL-positive.
Antibiotic treatment: The mean (± SD) hospitalization length was 11.21 ± 8.15 days (median 9 days, range 5-44 days). The most commonly used antibiotics used for the empiric treatment of UTI infants were ceftriaxone (20 patients, 17.1%, 18 as single therapy and 2 in combination therapy with gentamicin), ampicillin (17, 14.1%, 5 in combination with gentamicin and 3 with cefuroxime, the other 9 as single therapy in patients with an initial negative dipstick examination), gentamicin (16, 13.7%, 4 as single therapy), cefuroxime (15, 12.8%, 10 as single therapy), colistin (10, 8.5%, 7 as single therapy) and ceftazidime (10, 8.5%, all as single therapy).
Twelve (10.3%) patients were started on antibiotic therapy only after the urine culture results were available. The initial antibiotic therapy was modified after the urine culture and pathogen susceptibility was available in 32 (27.4%) patients.
Antibiotic prophylaxis was prescribed for 23 (10.7%) infants; ceftibuten (14 patients, 12%) and cefaclor (5, 4.3%) were the most frequently used antibiotic drugs.
All 5 Proteus spp. tested were nonsusceptible to ampicillin, TMP/SMX, gentamicin and chloramphenicol and susceptible to cefuroxime, ceftriaxone and nalidixic acid.
Discussion
The purpose of the present study was: 1) To describe in detail the first UTI episode in infants <3 months of age, in order to determine its demographic, epidemiologic, microbiological and therapeutic characteristics; 2) To describe the antibiotic susceptibility patterns of the major uropathogens isolated during the study period; and 3) To discuss the local antibiotic treatment policies for the treatment of UTI in young infants in the perspective of local microbiological data and medical literature recommendations.
The pediatric division of the Brasov Children’s Clinic Hospital admitted during each year of the study period around 30 young infants <3 months of age with the diagnosis of UTI. We found that: 1. UTI prevalence was high (12%) and E. coli and Klebsiella spp. were the main uropathogens isolated, without significant differences between patient gender and age subgroups; 2. UTI in the study population was characterized by low percentages of febrile infants at admission, absence of urosepsis, low percentage of leukocytosis or neutropenia in the peripheral blood counts and normal renal ultrasound examinations; 3. Very high resistance rates to 2nd and 3d generation cephalosporins, ciprofloxacin and gentamicin were recorded among the E. coli and Klebsiella spp. isolates, while piperacillin/tazobactam, meropenem, nalidixic acid, chlomphenicol and colistin retained good susceptibility rates.
Antibiotic treatment is the cornerstone of treatment for acute urinary tract infections and is important for preventing parenchymal localization of the infection [1,3,4,8-10]. When intravenous treatment is required, no particular antibiotic has been shown to be superior; cephalosporins and aminoglycosides are frequently recommended [1,3,4,9]. Bracy et al. summarized in 2016, in a systematic review and metaanalysis, 58 observational studies investigating the prevalence of antibiotic resistance among 77783 E. coli isolates in UTIs in children in primary care stratified by the OECD (Organization for Economic Co-operation and Development) [14] . In studies from industrialized countries, the pooled prevalence of resistance was 53.4% (95% confidence interval 46.0% to 60.8%) for ampicillin, 23.6% (13.9% to 32.3%) for trimethoprim, 8.2% (7.9% to 9.6%) for co-amoxiclav, and 2.1% (0.8 to 4.4%) for ciprofloxacin, while nitrofurantoin was the lowest at 1.3% (0.8% to 1.7%). Resistance in studies in among children in developing nations was significantly higher: 79.8% (73.0% to 87.7%) for ampicillin, 60.3% (40.9% to 79.0%) for co-amoxiclav, 26.8% (11.1% to 43.0%) for ciprofloxacin and 17.0% (9.8% to 24.2%) for nitrofurantoin. Bacterial isolates from the urinary tract from individual children who had received previous prescriptions for antibiotics in primary care were more likely to be resistant to antibiotics, and this increased risk could persist for up to six months [14]. Several studies performed around the world in pediatric patients emphasize an increasing rate of resistance of the main UTI pathogens (E. coli and Klebsiella spp.) to oral antibiotics prescribed as first-line of therapy in communityacquired disease (like amoxicillin, TMP/SMX and amoxicillin/ clavulanate) and, in lower percentages, oral cephalosporins, gentamicin and ciprofloxacin [12-19]. Furthermore, the prevalence of urinary tract infections caused by extensivespectrum β lactamase (ESBL)-producing organisms (particularly E. coli and Klebsiella spp.) is in continuous increase [15,20-22].
Information on antibiotic resistance patterns of Gram-negative organisms in Romania is limited. According to the European Union reports, 22% of E. coli strains analyzed from Romania are resistant to 3rd generation cephalosporins (10.9% being multiple-drug resistant), while 44% of K. pneumoniae strains were resistant were resistant to 3rd generation cephalosporins (30% exhibiting multiple-drug resistance) [23]. Recently, a microarray experimental model was used to detect the expression of 31 antimicrobial resistance genes of 75 ESBL producing-E coli and 66 ESBL producing-K. pneumoniae isolates obtained from clinical samples (both community and hospital-acquired) in Western Romania [24]. The authors reported that all hospital-acquired and 54% of community acquired E. coli strains had at least one resistance gene to 3rd generation cephalosporins, while the respective figures for K. pneumoniae were 81% and 6%, respectively [24]. We showed, in the present study, that the prevalence of resistance of E. coli and Klebsiella spp. to commonly prescribed intravenous antibiotics and the emergence of ESBL in hospitalized young infants diagnosed with UTI in Brasov, Central Romania, were extremely high and of extreme concern. The possible explanations reside, most probably, in the excessive therapeutic overuse and abuse of antibiotic drugs, at the community and at the hospital level as well, rending many antibiotics ineffective, at least as first line treatments for urinary tract infection.
The limitations of the present study are related, obviously, to its retrospective nature, which may have caused to some missing or inexact information in the data collection from the medical and laboratory charts. The fact that the number of febrile infants with UTI was relatively low (28.2% of all enrolled patients) may be explained by the presentation of body temperature values recorded at admission only, possibly after administration of antipyretics at home or during transportation to the PER. Another limitation is related to the relatively small number of tests performed for intravenous broad-spectrum antibiotics (like piperacillin/tazobactam or meropenem) and amikacin, not allowing a definitive picture for the susceptibility patterns of these antibiotics which may be needed as possible replacers for the empiric first-liners like ampicillin, gentamicin, amoxicillin/clavulanate or cefuroxime.
In conclusion, the resistance to antibiotics of E. coli and Klebsiella spp., the main uropathogens recovered in young infants with UTI in our hospital, was extremely high and reflects an important public health in the whole country. Facing this concerning situation, implementing close monitoring and rotating antibiotic drugs protocols together with a policy of modifying or changing the existing empiric antibiotic treatment protocols according to available epidemiologic local information, may represent logical and efficacious solutions.
References
- Roberts KB. Urinary tract infection treatment and evaluation. Update. Pediatr Infect Dis J 2004; 23: 1163-1164.
- Shah G, Upadhyay J. Controversies in the diagnosis and management or urinary tract infections in children. Pediatr Drugs 2005; 7: 339-346.
- Marcus N, Ashkenazi S, Yaari A, Samra Z, Livni G. Non-Escherichia coli versus Escherichia coli community-acquired urinary tract infection in children hospitalized in a tertiary center- relative frequency, risk factors, antimicrobial resistance and outcome. Pediatr Infect Dis J 2005; 24: 581-585.
- Ismaili K, Wissing KM, Lolin K, Le QK, Christophe C, Lepage P, Hall M. Characteristics of first urinary tract infection with fever in children- a prospective clinical and imaging study. Pediatr Infect Dis J 2011; 30: 371-374.
- Hoberman A, Wald ER, Reynolds EA, Penchansky I, Charron M. Is urine culture necessary to rule out urinary tract infection in young febrile children? Pediatr Infect Dis J 1996; 15: 304-308.
- Shaw KN, Gorelick M, McGowan KL, Yakscoe NM, Schwartz JS. Prevalence of urinary tract infection in febrile young children in the emergency department. Pediatrics 1998; 102: e16.
- Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J 2008; 27: 302-308.
- American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics 1999; 103: 843-852.
- Subcommittee on Urinary Tract Infection, Steering committee on Quality Improvement and Management. Urinary Tract Infection: Clinical Practice Guideline for the Diagnosis and Management of the Initial UTI in Febrile Infants and Children 2 to 24 months. American Academy of Pediatrics. Pediatrics 2011; 128: 595-610.
- Finnell SME, Carroll AE, Downs SM. Technical report- diagnosis and management of an initial UTI in febrile infants and young children. American Academy of Pediatrics. Pediatrics 2011; 128: e749-e770.
- Stein R, Dogan HS, Hoebeke P, Kocvara R, Nijman RJM, Radmayr C, Tekgul S. Urinary tract infections in children: EAU/ESPU guidelines. European Urology 2015; 67: 546-558.
- Zhanel GG, Hisanaga TL, Laing NM, DeCorby MR, Nichol KA, Palatnik LP, Johnson J, Noreddin A, Harding GK, Nicolle LE, Hoban DJ; NAUTICA Group. Antibiotic resistance in outpatient urinary isolates; final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents 2005; 25: 380-388.
- Gaspari RJ, Dickson E, Karlowsky J, Doern G. Antibiotic resistance trends in paediatric uropathogens. Int J Antimicrob Agents 2005; 26: 267-271.
- Bryce A, Hay AD, Lane IF, Thornton HV, Wootton M, Costelloe C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. BMJ 2016; 352: i939.
- Topaloglu R, Er I, Dogan BG, Bilginer Y, Ozaltin F, Besbas N, Ozen S, Bakkaloglu A, Gur D. Risk factors in community-acquired urinary tract infections caused by ESBL-producing bacteria in children. Pediatr Nephrol 2010; 25: 919-925.
- Caracciolo A, Bettinelli A, Bonato C, Isimbaldi C, Tagliabue A, Langoni L, Bianchetti MG. Antimicrobial resistance among Escherichia coli that cause childhood community-acquired urinary tract infections in Northern Italy. Italian J Pediatr 2011; 37: 3.
- Wu JH, Chiou YS, Chang JT, Wang HP, Chen YY, Hsieh KS. Urinary tract infections in infants: A single-center clinical analysis in southern Taiwan. Pediatr Neonatol 2012; 53: 283-288.
- Abuhandan M, Guzel B, Oymak Y, Ciftci H. Antibiotic sensitivity and resistance in children with urinary tract infection in Sanliurfa. Turkish J Urol 2013; 39: 106-110.
- Mehr SS, Powell CV, Curtis N. Cephalosporin resistant urinary tract infections in young children. J Paediatr Child Health 2004; 40: 48-52.
- Manges AR, Johnson JR, Foxman B, O'Bryan TT, Fullerton KE, Riley LW. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N Engl J Med 2001; 345: 1007-1013.
- Rawat D, Nair D. Extended-spectrum beta-lactamases in Gram negative bacteria. J Glob Infect Dis 2010; 2: 263-274.
- Alyamani EJ, Khiyami AM, Booq RY, Majrashi MA, Bahwert FS, Rechkina E. The occurrence of ESBL-producing Escherichia coli carrying aminoglycoside resistance genes in urinary tract infections in Saudi Arabia. Ann Clin Microbiol Antimicrob 2017; 16: 1-13.
- EARSS. Antimicrobial resistance surveillance in Europe 2011.
- Licker M, Anghel A, Moldovan R, Hogea E, Muntean D, Horhat F, Seclaman E, Tamas L, Anghel M, Baditoiu L. Genotype-phenotype correlation in multiresistant Escherichia coli and Klebsiella pneumoniae strains isolated in Western Romania. Eur Rev Med Pharmacol Sci 2015; 19: 1888-1894.