ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 10
Flavonoids of Fructus aurantii processed using the Zhangband system
Yi-hao Jiang1,2*, Dai-quan Jiang1, Peng-fei Wang1, Xue Wang1 and Zhi-baio Yu1
1School of Environmental and Chemical Engineering Resources, Nanchang University, Nanchang, PR China
2Jiangxi Province Key Laboratory of Edible and Medicinal Plant Resources, Nanchang University, Nanchang, PR China
- *Corresponding Author:
- Yi-hao Jiang
School of Environmental and Chemical Engineering Resources
Nanchang University, PR China
Accepted date: February 22, 2017
Background: To analyse the flavonoid components of Fructus Aurantii processed using the Zhangband method. Fructus Aurantii, the dried immature fruit of Citrus aurantium L., achieves its highest medicinal efficacy using the Zhangband method of processing, a specialized system of traditional Chinese medicine developed in Jiangxi Province, China. The flavonoids responsible for the medicinal qualities have never been analysed.
Methods: Fructus aurantii was processed according to traditional methods. Flavonoids were isolated by chromatography and identified spectrophotometrically.
Results: Four principal flavonoids were isolated: naringin (I), hesperindin (II), neohesperindin (III), and hesperetin 7-O-β-D-glucopyranoside (IV).
Conclusions: Hesperetin 7-O-β-D-glucopyranoside was newly identified in Fructus Aurantii; the compound was present in Fructus aurantii after, but not before, processing using the Zhangband method. Processing appears to attenuate the drying characteristics of Fructus aurantii and to enhance its medicinal efficacy.
Keywords
Fructus aurantii, Processing, Chemical components, Zhangband method, Flavonoids, Chinese medicine.
Introduction
Fructus Aurantii (FA), the dried immature fruit of Citrus aurantium L. and cultivars thereof, is a medicinal herb produced locally in Jiangxi Province, China. The fruit is bitter, sour, and pungent, and the processed herb has a warming effect on the body; FA acts on the spleen, stomach, and large intestine to regulate and activate Qi and relieve distention. Clinically, FA is generally used to treat Qi stagnation in the chest, intestinal distention and pain, dyspepsia, phlegm and fluid retention, gastroptosis, uterine prolapse, and rectocele. In recent years, FA has been found to be effective in the treatment of gallstones [1], cancer [2], anxiety [3], diabetes [4], and obesity [5], and thus shows a broad range of applications. Fructus Aurantii contains principally alkaloids, flavonoids, and volatile oils, with the flavonoids being the most important ingredients medically. In addition, FA contains smaller amounts of coumarins, tannins, saccharides, organic acids, and microelements.
Medicinal herbs, including those derived from FA, are abundant in Jiangxi Province, and the region has a long history of involvement in traditional Chinese medicine. The famous herb-processing system known as the Zhangband system was established in the region of Zhangshu, and it is said that the medicinal herbs processed in Zhangshu using the Zhangband system are particularly powerful and efficacious. The Chinese herbs processed by the Zhangband method are characterized by unique colours and remarkable levels of efficacy, and they are famous worldwide. Details of the Zhangband method, which is unique to Jiangxi Province, are known to only a small number of advanced herbal technicians who are considered as masters of the technique. Moreover, the long-term survival of the system is uncertain, as it depends on the passage of the techniques from one generation to the next. In addition, no systematic study of the herbs using modern methodologies has yet been conducted, and quality control of processed herbs is based solely on the experience of the herbal technicians.
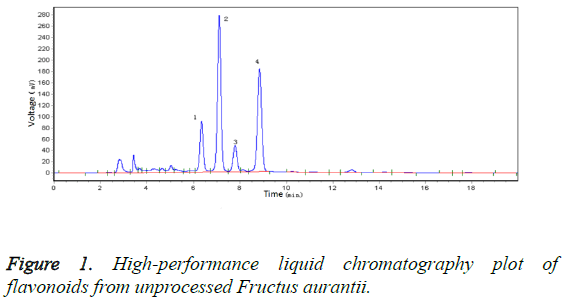
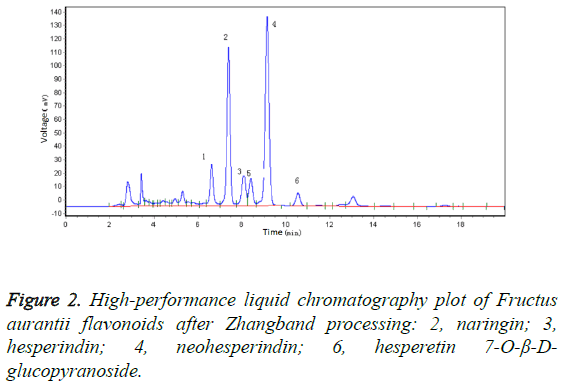
After Zhangband processing, the skin of FA is green, and the fruit contains a thick pulp that is whitish in colour and has a strong flavour and a small pulp sac in the shape of a chrysanthemum. The fruit is of high quality and is very efficacious, being the best form of FA available. In this study, we compared the FA materials before and after Zhangband processing using high-performance liquid chromatography (Figure 1), which showed that two new peaks (peaks 5 and 6, Figure 2) developed during processing. We identified the substance of peak 6 as hesperetin7-O-β-D-glucopyranoside.
Materials and Methods
Instruments and reagents
Samples were analysed using: a rotary evaporator (model RF-52; Shanghai Ya-rong Biochemical Instruments); an X-4 digital melting point microdetector (Beijing Tech Instrument Co. Ltd.); a magnetic resonance spectrometer (Bruker av400; Switzerland); D101 large-pore adsorbent resin (Cangzhou Bon Adsorber Technology Co. Ltd., catalogue no. 080912); 200-300 mesh silica gel for column chromatography (Qingdao Yinhai Chemicals); 60-90 mesh polyamide 20080331 (Yangyu, Hengjie Town, Lu-qiao District, Taizhou City, Zhejiang Province); silica gel plates with dimensions of 100 × 100 mm, 100 × 200 mm, and 100 × 50 mm (Qingdao Yinhai Chemical Co.); 10 × 20 cm of polyamide film (Taizhou Lu-qiao Sijia Biochemical Plastics). All reagents were analytically pure.
Herbal materials
Fructus Aurantii material was purchased from Jiangxi Tianqitang Pharmaceuticals Co. Ltd. and was confirmed to be the dried immature fruit of Citrus aurantium L. by Professor Liu Qing-hua of the Chinese Herb Identification Laboratory, Jiangxi University of Traditional Chinese Medicine, Jiangxi, China.
Zhangband processing of FA
Pulp was removed from FA using a knife, and was washed with water to remove sand and mud. The pulp was collected and moistened overnight (12 h), flattened using an iron weight, and subject to further pressure using a specially designed frame for 3-5 days. When mould spots started to develop, the pressed cake was folded in half and sliced into pieces in the shape of a “phoenix eye”; the pieces were then sun dried. Next, wheat bran was placed into a pot and, when whitish smoke arose upon heating, dried FA pieces were added and stirred continuously until they became light yellow in colour. The FA pieces were then removed from the pot, allowed to cool, and the scorched bran was mesh-filtered. The filtrate was added to the cooled FA pieces. A total of 5-10 kg of wheat bran was used to process each 100 kg batch of FA pieces.
Extraction and isolation
Pieces of FA (mass, 2 kg) were coarsely ground and extractions were performed twice, for 2 h each time, using 70% (v/v) ethanol at a ratio of 1:8 FA:ethanol (v/v). The extracts were combined and evaporated to dryness under reduced pressure. The dried powders were dissolved in water, absorbed into D-101 large-pore resin, and eluted with water to remove soluble impurities; elutions were performed sequentially using 10%, 30%, 50%, 70%, and 95% (v/v) ethanol. The fractions were combined, evaporated to dryness, re-dissolved in water, re-absorbed into the resin, re-eluted with 50% (v/v) ethanol, and subjected to silica gel column chromatography.
Results: Structural Identifications
Silica gel column chromatography yielded four compounds: naringin (compound I), hesperindin (compound II), neohesperindin (compound III), and hesperetin 7-O-β-Dglucopyranoside (compound IV).
Compound I: naringin
A white powder collected upon evaporation of methanol, mp 238-240?C; AlC13 significantly enhanced the fluorescence intensity of the material. 1H-NMR (DMSO-d6, 400 MHz); δ: 11.96 (1H, s, 5-OH), 9.55 (1H, s, 4-OH), 7.24 (2H, d, J=8.24 Hz, H-3’, 5’), 6.70 (2H, d, J=7.72 Hz, H-2’, 6’), 6.02 (1H, s, H-6), 5.99 (1H, s, H-8), 5.25 (1H, d, J=4.6 Hz, H-2), 3.3 (1H, d, J=9.6 Hz, H-3), 2.62 (1H, d, J=9.6 Hz, H-3), 5.06 (1H, d, J=5.04 Hz, H-1’’), 5.0 (1H, s, H-1’’’), 1.05 (3H, d, J=5.64 Hz, 6’’’-CH3). 3C-NMR (DMSO-d6, 100 MHz); δ: 78.77 (C-2), 42.06 (C-3), 197.23 (C-4), 162.88 (C-5), 96.25 (C-6), 164.81 (C-7), 95.07 (C-8), 162.7 (C-9), 103.27 (C-10), 128.51 (C-1’), 128.4 (C-2’), 115.17 (C-3’), 157.76 (C-4’), 115.17 (C-5’), 128.4 (C-6’), 100.36 (C-1”), 76.8 (C-2”), 78.58 (C-3”), 69.55 (C-4”), 77.07 (C-5”), 60.4 (C-6”), 97.25 (C-1”’), 70.43 (C-2”’), 70.33 (C-3”’), 71.77 (C-4”’), 68.25 (C-5”’), 18.0 (C-6”’). These data indicate that the compound is naringin [6].
Compound II: hesperindin
A white powder on evaporation of absolute ethyl alcohol, mp 256-263?C; AlC13 significantly enhanced the fluorescence intensity of the material. 1H-NMR (DMSO-d6, 400 MHz); δ: 12.03 (1H, s, 5-OH), 9.15 (1H, s, 3’-OH), 6.95-6.89 (3H, m, H-2’ , 5’, 6’), 6.14 (1H, s, H-6), 6.12 (1H, s, H-8), 5.44 (1H, d, J=4.8 Hz, H-2), 3.28 (1H, d, J=4.5 Hz, H-3), 3.14 (1H, d, J=5.76 Hz, H-3), 3.77 (3H, s, -OCH3), 1.07 (3H, d, J=7.4 Hz, 6’’’-CH3); 13C-NMR (DMSO-d6, 100 MHz); δ: 78.32 (C-2), 41.99 (C-3), 196.97 (C-4), 162.98 (C-5), 96.34 (C-6), 165.08 (C-7), 95.51 (C-8), 162.44 (C-9), 103.28 (C-10), 130.84 (C-1’), 114.08 (C-2’), 146.39 (C-3’), 147.92 (C-4’), 112.0 (C-5’), 117.93 (C-6’), 99.41 (C-1’’), 75.47 (C-2’’), 72.02 (C-3’’), 69.56 (C-4’’), 76.2 (C-5’’), 65.97 (C-6’’), 100.54 (C-1’’’), 70.22 (C-2’’’), 70.65 (C-3’’’), 72.94 (C-4’’’), 68.27 (C-5’’’), 17.77 (C-6’’’), 55.65 (C-OCH3). These data indicate that the compound is hesperindin [7].
Compound III: neohesperindin
A white powder upon evaporation of absolute ethyl alcohol, mp 246-247?C; AlC13 significantly enhanced the fluorescence intensity of the material. 1H-NMR (DMSO-d6, 400 MHz) δ: 12.05 (1H, s, 5-OH), 9.17 (1H, s, 3’-OH), 6.95-6.87 (3H, m, H-2’, 5’, 6’), 6.11 (1H, s, H-6), 6.09 (1H, s, H-8), 3.77 (3H, s, 4OCH3), 5.36 (1H, d, J=6.84 Hz, H-2), 3.38 (1H, s, H-3), 2.51 (1H, s, H-3), 5.17 (1H, d, J=5.32 Hz, H-1’’), 5.1 (1H, s, H-1’’’), 1.16 (1H, d, J=6.2 Hz, H-6’’’); 13C-NMR (DMSO-d6, 100 MHz); δ: 76.53 (C-2), 41.56 (C-3), 196.41 (C-4), 162.33 (C-5), 95.68 (C-6), 164.25 (C-7), 94.57 (C-8), 162.0 (C-9), 102.75 (C-10), 130.3 (C-1’), 113.52 (C-2’), 145.89 (C-3’), 147.41 (C-4’), 111.44 (C-5’), 117.25 (C-6’), 96.83 (C-1’’), 77.83 (C-2’’), 76.3 (C-3’’), 67.7 (C-4’’), 75.53 (C-5’’), 59.84 (C-6’’), 99.81 (C-1’’’), 69.78 (C-2’’’), 99.81 (C-3’’’), 71.22 (C-4’’’), 69.0 (C-5’’’), 17.43 (C-6’’’), 55.1 (C-OCH3). These data indicate that the compound is neohesperindin [8].
Compound IV: hesperetin 7-O-β-D-glucopyranoside
A white powder upon evaporation of absolute ethyl alcohol, mp 223-225?C; AlC13 significantly enhanced the fluorescence intensity of the material. 1H-NMR (DMSO-d6, 400 MHz); δ: 12.05 (1H, s, 5-OH), 9.16 (1H, s, 3’-OH), 6.96-6.87 (3H, m, H-2’, 5’, 6’), 6.16 (1H, s, H-6), 6.14 (1H, s, H-8), 5.50 (1H, dd, J=3.97 Hz, H-2), 2.76 (1H, d, J=14.5 Hz, H-3), 3.29 (1H, d, J=12.9 Hz, H-3), 3.76 (3H, s, 4’-OCH3), 4.97 (1H, d, J=7.41 Hz, H-1’’); 13C-NMR (DMSO-d6, 100 MHz); δ: 78.43 (C-2), 42.13 (C-3), 196.98 (C-4), 162.9 (C-5), 96.46 (C-6), 65.28 (C-7), 95.46 (C-8), 162.57 (C-9), 103.26 (C-10), 130.89 (C-1’), 112.01 (C-2’), 147.95 (C-3’), 146.47 (C-4’), 114.13 (C-5’), 117.77 (C-6’), 99.59 (C-1’’), 73.0 (C-2’’), 76.29 (C-3’’), 69.49 (C-4’’), 77.07 (C-5’’), 60.55 (C-6’’), 55.68 (C-OCH3). These physicochemical characteristics and spectrophotometric data indicate that the compound is hesperetin 7-O-β-Dglucopyranoside [9].
Discussion and Conclusions
Four flavonoids were isolated from Zhangband-processed FA using large-pore adsorbent resin and repeated silica gel column chromatography [10,11]. Of these, hesperetin7-O-β-Dglucopyranoside was present after, but not before, Zhangband processing, and is herein described for the first time from FA.
Disorders of gastrointestinal motility are important features of Qi stagnation of the spleen and stomach. Fructus aurantii effectively regulates Qi and relieves stagnation in these areas, and is also used to treat Qi stagnation of the chest and abdomen, abdominal distention, and dyspepsia. However, raw FA exerts excessive drying of tissues. Pharmacological experiments have shown that a decoction of FA processed using the Zhangband method exhibits milder effects on ex-vivo rabbit intestines and the gastrointestinal motility of live mice, as compared to raw FA. Thus, processing attenuates the drying characteristics of FA and enhances the ability of the material to strengthen the spleen and stomach. Such changes in the pharmacological effects of processed FA may be associated with chemical changes that occur during processing. Thus, the present study provides evidence that Zhangband processing may change the pharmacological actions of FA.
Acknowledgments
None
Declaration of Interest
The authors report no declarations of interest.
References
- Chang HM, But PP, Yao SC, Wang LL, Yeung SCS. Pharmacology and applications of Chinese materia medica. World Sci Publ Singapore 1986; 15: 73-77.
- Arias BA, Ramon-Laca L. Pharmacological properties of citrus and their ancient and medieval uses in the Mediterranean region. J Ethnopharmacol 2005; 97: 89-95.
- Pultrini Ade M, Galindo LA, Costa M. Effects of the essential oil from Citrus aurantium L. in experimental anxiety models in mice. Life Sci 2006; 78: 1720-1725.
- Campbell JI, Mortensen A, Mrlgaard P. Tissue lipid lowering-effect of a traditional Nigerian antidiabetic nfusion of Rauwolfia vomitoria foilage and Citrus aurantium fruit. J Ethnopharmacol 2006; 104: 379-386.
- Haller CA, Benowitz NL, Jacob P. Hemodynamic effects of ephedra-free weight-loss supplements in humans. Am J Med 2005; 118: 998-1003.
- Fu XM, Wu ZHG, Chu XL. Study on the flavonoids in Fructus aurantii. Chinese Herb Med 2006; 29: 1187.
- Zhang ZF, Bian BL, Yang J, Tian XF. Studies on chemical constituents in roots of Jasminum sambac. Zhongguo Zhong Yao Za Zhi 2004; 29: 237-239.
- Liu YH, Wu XY, Fang JG, Tang J. Study on the chemical constituents of Radix isatidis. Chin Trad Herb Drug 2003; 34: 777.
- Wang YJ, Yang XW, Guo QS. Study on the chemical constituents of Yellow chrysanthemums. China J Chinese Materia Medica 2008; 33: 526.
- Gong QF, Zhang S. Traditional Chinese medicine processing book. Nanchang Jiangxi Science and Technology Publishing Press 1991: 238.
- Ma Y. The gastrointestinal effects of Fructus aurantii and the changes before and after processing. Pharmacol Clinics TCM 1996; l2: 28-29.