ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 7
Feasibility of biventricular resynchronization through left univentricular pacing beat-by-beat tracking of physiological atrioventricular delay
Department of Cardiology, the First Affiliated Hospital of Kunming Medical University, Institute of Cardiovascular Diseases, Kunming, Yunnan province, China
- *Corresponding Author:
- Tao Guo
Department of Cardiology
The First Affiliated Hospital of Kunming Medical University
Institute of Cardiovascular Diseases, China
Accepted date: December 5, 2016
This study aimed to investigate the feasibility of Cardiac Resynchronization Therapy (CRT) achieved through Left Univentricular Pacing (LUVP) Beat-By-Beat Tracking (BBBT) of physiological Atrioventricular Delay (AVD). Thirty-seven patients undergoing CRT were enrolled, and their preoperative atrial premature conduction data were collected. Electrocardiogram (ECG) was used to optimize the LUVP, as well as the AVD, VV, and VR intervals of standard biventricular pacing. The interval from the LUVP pulse to the start of Right Ventricular (LVP-RV) intracavitary ECG was measured, and the LV priority coefficient (ε) was calculated. The interval differences between two adjacent sinus heartbeats within 1 min, as well as between the maximum and minimum Atrial Sensing- Ventricular Sensing (AS-VS), were measured and compared. The regression equation for atrial premature AVD was P'R'=0.022+0.954 PR. The LVP-RV interval (102.6 ± 15.8 ms) was significantly greater than the V-R interval (19.31 ± 7.32 ms) (P<0.01), and ε was 0.73 ± 0.04. The maximum difference between the AS-VS intervals of two adjacent sinus heartbeats within 1 min (6.43 ± 1.63 ms) was significantly lower than the difference between the maximum and minimum AS-VS intervals (16.54 ± 3.32 ms) (P<0.01). LUVP-BBBT could realize CRT.
Keywords
Chronic congestive heart failure, Cardiac resynchronization therapy, Left univentricular pacing
Introduction
Chronic Congestive Heart Failure (CHF) is often accompanied by Complete Left Bundle Branch Block (CLBBB), leading to the dyssynchrony of the Left Ventricular (LV) and Right Ventricular (RV) constrictions, and reduction in effective cardiac output [1,2]. Although Cardiac Resynchronization Therapy (CRT) has achieved exact effects [3], its short and fixed Atrioventricular Delay (AVD) to ensure biventricular capture [4] lacks physiological atrioventricular node conduction; thus, it does not meet the physiological requirements. Furthermore, the RV pacing-induced agitation would be conducted slowly, unevenly, and conversely to the His-Purkinje system (HPS) through the cardiac muscles, this is against the physiologies of agitation conduction. Thus, it could cause damage to the ventricular structures and functions, offset the benefits of CRT, or even partially explain why partial CHF patients were unresponsive to CRT [5-7].
Generally, CHF patient with CLBBB have normal atrioventricular node and right HPS (rHPS) conduction; thus, the right ventricle does not need electrical pacing [8]. This suggests that the fusion of Left Univentricular Pacing (LUVP) and rHPS down conducted self-agitations could achieve Biventricular Resynchronization (BVR) and retain physiological AVD to coordinate the atrium-induced ventricular filling [9,10], in which the adaptive CRT algorithm [11] could measure the interval of Atrial Sensing-Ventricular Sensing (AS-VS) once per minute: if ≤ 200 ms, 70% of this interval would be used as the pacemaker-programmed AVD; if>200 ms, it would be converted to standard Biventricular Pacing (BVP). However, this algorithm has the following limitations: first, the once-per-minute AVD extension would allow self-agitations to be conducted from the rHPS to the RV, and although it could open the Ventricular Sensing Reaction (VSR) [12], LV agitation would still be delayed. Even through calculation with the lower rate of 60 beats/min, LV priority could not be achieved for 24 min in 1 or 6 days in 1 year. If the battery life of a three-chamber pacemaker is set as 5 years, BVR could not be achieved for 1 month. Second, the pacemaker-programmed AVD is fixed in each minute; when a patient needs to convert between rest and exercise within this minute, the heart rate and physiological AVD would change accordingly [13,14], thus inevitably leading to the nonsynchronization of LUVP and rHPS down conducted physiological agitations, as well as an uneven LV priority, which both could partially offset the benefits of CRT. Theoretically, the difference between the physiological AVD of two adjacent heartbeats is small and could be ignored, suggesting that exploring one LUVP-BBBT algorithm could achieve BVR and help in overcoming the above limitations.
Materials and Methods
Subjects
Thirty-seven CHF patients admitted to the Department of Cardiology, First Affiliated Hospital of Kunming Medical University, from May 2013 to November 2015, were selected. These patients all met the class I/A indications of CRT guidelines [15], namely optimal drug treatment-based cardiac functions>class I, sinus rhythm, left bundle branch block, QRS wave duration>150 ms, and LV Ejection Fraction (LVEF) ≤ 35%. All patients signed the informed consent form for threechamber pacemaker implantation, and agreed to participate in this study. Their mean age was 55 ± 13 years; 22 were men and 15 were women. The exclusion criteria were as follows: (i) atrioventricular block, (ii) expected survival period<1 year, (iii) potential reversible cardiomyopathy, and (iv) valvular heart diseases. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Kunming Medical University. Written informed consent was obtained from all participants.
CRT pacing system
The three-chamber pacemakers (CRT-P/D) used in this study included D394TRG, D284TRK, and C2TR01 (Medtronic Co., USA); P107 P053 (Boston Scientific Co., USA); Stratos LV and Lumax300HF-T (Biotronik, Germany); and V-350, CD3211-36Q, CD3231-40, and CD3249-40 (St. Jude Medical, USA). The implantation was performed through conventional standard methods.
Sampling the atrial premature template
Dynamic Electrocardiogram (ECG) was performed 24 h before surgery to sample the atrial premature template; thereafter, by using the atrial premature P'R' interval (ap-P'R') as the dependent variable, and the previous sinus heartbeat PR interval (ps-PR) and the interval from the P-wave of this sinus heartbeat to the P' of the atrial premature (P-P' interval) as the independent variables, multiple linear regression (stepwise) was used to establish the PR and P-P' interval-derived regression equation of physiological AVD when atrial premature conduction occurred.
Detection of ECG parameters
A Vivid E9 cardiac color ultrasound imaging device (GE, USA) was used with a probe transmit frequency of 2.5 MHz. Besides conventional ECG parameters, the following indicators were also measured: (i) mitral regurgitation area (MRA); (ii) LVEF; (iii) aortic forward blood flow velocitytime integral (AVI); (iv) intraventricular synchronization parameters: intervals before LV and RV ejections, namely intervals before aortic and pulmonary arterial ejection (intervals starting from QRS and reaching the aortic and pulmonary arterial flow spectra, respectively), calculating the interval difference between RV and LV ejections (i.e., Interventricular Mechanical Delay (IVMD)); and (v) intra-LV synchronization parameters: the tissue synchronization imaging technique was used to analyse the peaking times of 12 LV segments [16], namely the basal and middle segments of the lateral wall, basal and middle segments of the posterior interventricular septum, basal and middle segments of the inferior wall, basal and middle segments of the anterior wall, basal and middle segments of the posterior wall, and basal and middle segments of the anterior interventricular septum. The standard deviations of these 12 peaking times were also calculated (Ts-SD12) [17]. The above data were measured during three cardiac cycles and averaged.
Optimization of A-V and V-V during standard BVP
The programmed pacing mode was BVP, and AVI was measured after each AVD titration and pacing for 5 min. Meanwhile, referring to the mitral blood flow spectrum, the AVD corresponding to completely separated maximum peak E and A, minimum MRA, and maximum AVI was set as the optimized AV interval, and then the VV interval was also optimized to obtain the maximum VV interval corresponding to the AVI (as the optimized VV interval) [18].
LUVP optimization of LV before RV interval (V-R interval)
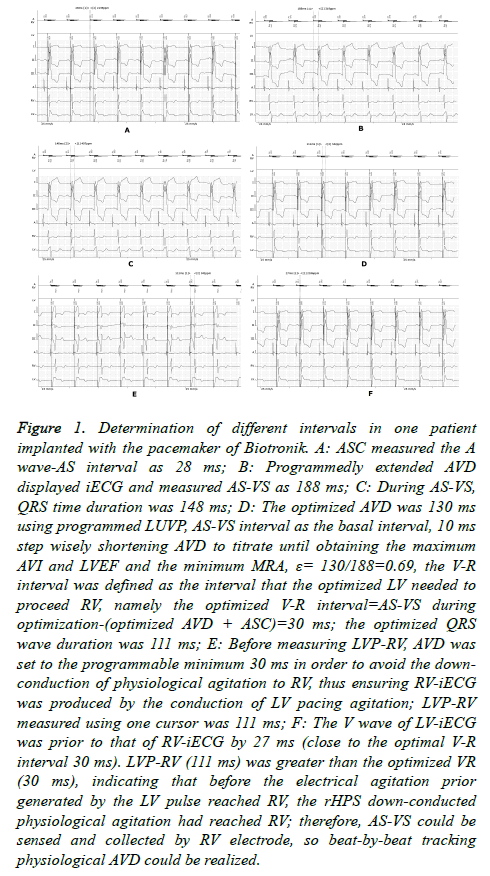
In this study, we defined the LUVP ECG-obtained interval that LV needed to precede RV as the optimized “V-R interval,” similar to the “V-V interval” in standard BVP. The program used LUVP, and AS Compensation (ASC) was performed to measure the interval from the start of the right atrial intracavitary ECG (iECG) A-wave to AS (Figure 1A). The AVD was then extended to display and measure the AS-VS interval (Figure 1B). By using this AS-VS interval-ASC as the basal interval, AVD was then shortened with a 10 ms step to titrate until obtaining the maximum AVI and LVEF, as well as the minimum MRA, and this AVD was named as the optimized AVD (Figure 1C); that is, when optimizing, the left AVD was the most optimal (during optimization, the patient’s heart rate was usually greater than the lower rate of the pacemaker; therefore, it was considered the sensed AV (SAV)). The formula for the optimized V-R interval was as follows: AS-VS interval-(optimized AVD+ASC) during optimization. In this study, we defined the percentage of the LUVP-optimized AVD to the physiological AVD, namely the ratio of optimized AVD/AS-VS interval during optimization, as the LV coefficient (ε) (Figure 1D).
Figure 1. Determination of different intervals in one patient implanted with the pacemaker of Biotronik. A: ASC measured the A wave-AS interval as 28 ms; B: Programmedly extended AVD displayed iECG and measured AS-VS as 188 ms; C: During AS-VS, QRS time duration was 148 ms; D: The optimized AVD was 130 ms using programmed LUVP, AS-VS interval as the basal interval, 10 ms step wisely shortening AVD to titrate until obtaining the maximum AVI and LVEF and the minimum MRA, ε= 130/188=0.69, the V-R interval was defined as the interval that the optimized LV needed to proceed RV, namely the optimized V-R interval=AS-VS during optimization-(optimized AVD + ASC)=30 ms; the optimized QRS wave duration was 111 ms; E: Before measuring LVP-RV, AVD was set to the programmable minimum 30 ms in order to avoid the downconduction of physiological agitation to RV, thus ensuring RV-iECG was produced by the conduction of LV pacing agitation; LVP-RV measured using one cursor was 111 ms; F: The V wave of LV-iECG was prior to that of RV-iECG by 27 ms (close to the optimal V-R interval 30 ms). LVP-RV (111 ms) was greater than the optimized VR (30 ms), indicating that before the electrical agitation prior generated by the LV pulse reached RV, the rHPS down-conducted physiological agitation had reached RV; therefore, AS-VS could be sensed and collected by RV electrode, so beat-by-beat tracking physiological AVD could be realized.
Determination of LVP-RV
AVD was program-extended to display and measure the ASVS, and then the program was set as LUVP and AVD was set as the programmable minimum (30 ms) in order to avoid the down conduction of physiological agitation to RV, thus ensuring RV-iECG was produced through the conduction of LV pacing agitation. The iECG was then captured, and LVP-RV was measured by using one cursor. The measurement results were saved in PDF format and restored to standard BVP for follow-up (Figure 1E and 1F).
Determination of the 1 min AS-VS interval difference
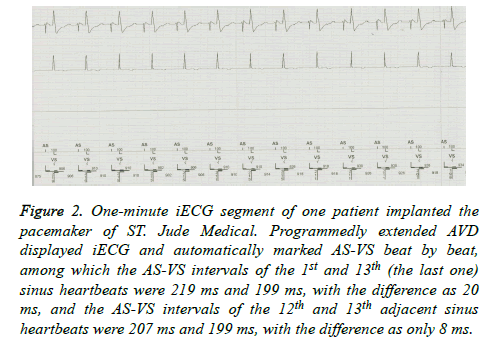
The AVD was program-extended until the iECG showed ASVS, and then the 1 min iECG was randomly captured and saved. The AS-VS between two adjacent sinus heartbeats within 1 min and the maximum and minimum AS-VS were then measured automatically or by using one cursor, and the differences between them were calculated (Figure 2).
Figure 2. One-minute iECG segment of one patient implanted the pacemaker of ST. Jude Medical. Programmedly extended AVD displayed iECG and automatically marked AS-VS beat by beat, among which the AS-VS intervals of the 1st and 13th (the last one) sinus heartbeats were 219 ms and 199 ms, with the difference as 20 ms, and the AS-VS intervals of the 12th and 13th adjacent sinus heartbeats were 207 ms and 199 ms, with the difference as only 8 ms.
Statistical analysis
SPSS17.0 statistical package was used for the analysis. Continuous variables are expressed as mean ± standard deviation (͞x ± s). Multiple linear regression was used to analyse the relationships among ps-PR, P-P', and ap-P'R'. Analysis of variance was used for intergroup comparison, and the t test was used for intragroup comparison of LVP-RV and optimized V-R. P<0.05 was considered statistically significant.
Results
Atrial premature template
The P-P' interval was not included into the regression equation (P=0.324), and the constant was 0.022 (P=0.02). The standardized partial regression coefficient of the PR interval was 0.954 (P=0.000); therefore, the PR interval-derived regression equation of physiological AVD when atrial premature conduction occurred was P'R'=0.022+0.954 PR.
Comparison of LUVP and standard BVP ECG optimization parameters
The MRA, AVI, IVMD, and QRS wave duration obtained by using LUVP were more improved than those obtained using the BVP (P<0.01 or 0.05); however, no significant difference was found in the LVEF and Ts-SD12 between the two modes (P>0.05) (Table 1).
| Parameter | Before CRT | BVP | LUVP |
|---|---|---|---|
| AVD (ms) | 176.5 ± 11.6 | 115.4 ± 6.1* | 128.6 ± 10.3*# |
| QRSwave duration (ms) | 181.2 ± 20.1 | 140.6 ± 11.2* | 134.8 ± 10.1*† |
| LVEF (%) | 0.26 ± 0.04 | 0.31 ± 0.06* | 0.33 ± 0.06* |
| MRA (cm2) | 4.33 ± 1.32 | 3.72 ± 1.23* | 3.15 ± 1.18*† |
| AVI (cm) | 15.72 ± 2.0 | 18.78 ± 1.0* | 19.41 ± 1.2*† |
| IVMD (ms) | 79.3 ± 14.2 | 72.4 ± 14.1‡ | 64.5 ± 13.3*† |
| Ts-SD12 (ms) | 105.5 ± 23.3 | 90.3 ± 21.2* | 86.4 ± 20.4* |
Table 1. Comparison of LUVP and standard BVP ECG optimization parameters.
V-R and LVP-RV
The ASC was 28.68 ± 2.24 ms, and LVP-RV (102.6 ± 15.8 ms) was significantly greater than V-R (19.32 ± 7.31 ms, P<0.01).
The AS-VS during optimization was 176.5 ± 11.6 ms, and the optimized AVD was 126 ± 10.31 ms, with ε being 0.73 ± 0.04.
The maximum difference of AS-VS between two adjacent sinus heartbeats within 1 min was 6.43 ± 1.63 ms, which is significantly less than the difference between the maximum and minimum AS-VS (16.54 ± 3.32, P<0.01).
Beat-by-beat tracking algorithm
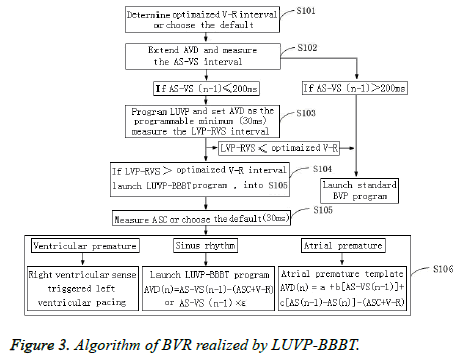
The Pacing AV (PAV) was SAV+ASC (Figure 3). If the n-1, n, and n+1 heartbeats were sinus heartbeats, their physiological AVD were AS-VS (n-1), AS-VS (n), and AS-VS (n+1), respectively, and the pacemaker-programmed LUVP-SAV n could select the VR interval method: SAV n=AS-VS (n-1)- (ASC+V-R); SAV (n+1)=AS-VS (n)-(ASC+V-R), or the LV priority coefficient method (ε): SAV n=AS-VS (n-1) × ε. Furthermore, ε could be individually calculated as the optimized AVD/AS-VS during optimization. In this study, ε= 0.73 ± 0.04, which is close to that in a previous report (0.7) [19], and n was the second heartbeat and any subsequent heartbeat after the heart pacemaker started working (n ≥ 2).
Atrial premature program
First, the atrial premature template was sampled: when the atrial electrode sensed atrial premature conduction, the pacing program set the AVD delay up to 300 ms and detected the AS'- VS' interval of the atrial premature beat. The AS-VS and ASAS' interval-derived regression equation of physiological AVD when atrial premature conduction occurred was then established by using the AS-VS of the pre-AS'-VS' sinus beat and the interval from AS to AS' of this sinus beat (AS-AS' interval) as the independent variables.
If the n-1 and n+1 heartbeats among the n-1, n, and n+1 heartbeats were sinus heartbeats, and the n heartbeat was an atrial premature beat, then its physiological AVD was AS-VS (n-1), AS-VS (n), and AS-VS (n+1), respectively, and the pacemaker-programmed LUVP-SAV n=AS'-VS'-(ASC+V-R); SAV (n+1)=AS-VS (n-1)-(ASC+V-R), among which AS'-VS' was the physiological AVD of this atrial premature conduction calculated by using the regression equation AS'-VS'=a+b (ASVS (n-1))+c (AS (n-1)-AS (n)), in which “a” was a constant, and “b” and “c” were standardized partial regression coefficients.
Ventricular premature program
If the n-1 and n+1 heartbeats among the n-1, n, and n+1 heartbeats were in sinus rhythm, and the n heartbeat was a ventricular premature beat, the physiological AVD of the n-1 and n+1 heartbeats was AS-VS (n-1) and AS-VS (n+1), respectively, and the VSR program of the n-heartbeat ventricular premature beat-started RV sensing trigger LUVP was SAV (n+1)=AS-VS (n-1)-(ASC+V-R).
Discussion
This study revealed that LUVP-optimized AVD did not show worse ECG parameters than did standard BVP in assessing hemodynamics and synchronization. In fact, some parameters were even better, consistent with recent reports [20], suggesting that the fusion of LUVP and rHPS down conducted agitation could achieve CRT, which not only retained the physiological AVD functions of the atrioventricular node but also restored the physiological agitation sequences of RV. Meanwhile, it corrected the non-physiological state caused by the RV apical pacing-agitated anti-HPS slow conduction among myocardial cells, thus demonstrating that it meets more physiological requirements than does standard BVP.
In this study, we used iECG to measure the intervals of LVPRV and V-R, and the results showed that the LVP-RV interval was significantly larger than the V-R interval. Therefore, before the pacing agitation previously generated by the LV pacing electrode was conducted to the right ventricle, selfagitations had been down conducted from the rHPS to the right ventricle, and the RV electrode could sample the AS-VS interval to act as the basis for calculating the next left AVD program of the heartbeat pacemaker. Furthermore, it did not need the adaptive CRT algorithm to extend the AVD each time for the AS-VS measurement. This study verified, for the first time, that on the premise of ensuring LV priority, the LUVP could track physiological AVD beat by beat, and establish one LUVP-BBBT algorithm. This algorithm was based on beat-bybeat AS-VS tracking with the pacemaker program, by using the pre-heartbeat AS-VS-(ASC+V-R) as the LUVP pacemakerprogrammed AVD of next heartbeat, thus fusing with rHPS down conducted physiological agitation and realizing CRT. When atrial premature conduction occurred, because its physiological AVD was related with the basic AVD of the previous sinus heartbeat and its coupling interval, the above intervals could be collected to establish the regression equation, which could be used later as the atrial premature template, as well as the pacemaker AVD program to realize BVR in the future. When ventricular premature conduction occurred, the RV sensing trigger LV pacing program could be started [8]. This program had been widely used in current three-chamber pacing systems and does not need further programming [12].
The results of this study showed that the difference between 1 min AS-VS was>10 ms, based on the fact that 10 ms is set as one level of ventricular interval in current programmable pacemakers. This suggests that the adaptive CRT algorithm might affect ventricular synchronization when patients convert between exercise and rest. However, the application of the LUVP-BBBT algorithm developed in this study made the ASVS interval difference between two adjacent heartbeats<10 ms, which is significantly less than that between the longest and shortest physiological AVD within 1 min in the adaptive CRT algorithm; thus, it would not significantly impact the LV and RV synchronization. Therefore, the LUVP-BBBT algorithm might be better than the adaptive CRT algorithm, and could solve the limitations of the adaptive CRT algorithm of not being able to track real-time physiological AVD, owing to obvious changes of AVD within 1 min and of the influence on the LV and RV synchronization.
In addition, because CRT usually needs LV priority, but the programmed left AVD of the pacemaker could not track realtime physiological AVD of the same heartbeat, the application of LUVP in tracking the physiological AVD of the previous heartbeat has almost reached the limits of “real-time” tracking of physiological AVD. Moreover, it could fuse the selfagitations normally conducted from the right bundle branches, thus recovering RV physiological atrioventricular conduction and RV physiological agitation sequences, thus meeting more physiological requirements and exhibiting important meaning toward improving the response rate to CRT. Meanwhile, the method did not need to extend the AVD to sample AS-VS, and it could be upgraded from the current per-minute optimization in the adaptive CRT algorithm to per-beat optimization, thus overcoming the needs of extending AVD every minute and affecting the limitations of biventricular synchronization. Therefore, it would help in developing a three-chamber pacemaker based on this algorithm, and in realizing physiological CRT. Furthermore, the application of this algorithm needed no electrical pacing of the RV electrode. Therefore, the three-chamber battery could only be used by two chambers, thus extending the battery life, reducing the average CRT costs, and reducing the economic burdens on the health insurance systems of developing countries, patients, and their families [8].
The new algorithm based on LUVP tracking of physiological AVD-achieved CRT would open the debate on whether CRT should keep or discard the physiological AVD functions of the atrioventricular node, and this might challenge the traditional concept of current CRT guidelines requiring 100% BVP [21].
Acknowledgments
This work was funded by National Natural Science Foundation of China (No: 81360044), Joint Special Foundation of Yunnan Provincial Science and Technology Department-Kunming Medical University (No: 2013FB133).
Conflict of Interest
All authors have no conflict of interest regarding this paper.
References
- Strik M, Regoli F, Auricchio A, Prinzen F. Electrical and mechanical ventricular activation during left bundle branch block and resynchronization. J Cardiovasc Transl Res 2012; 5: 117-126.
- Remme EW, Niederer S, Gjesdal O, Russell K, Hyde ER, Smith N, Smiseth OA. Factors determining the magnitude of the pre-ejection leftward septal motion in left bundle branch block. Europace 2015.
- Lalani GG, Birgersdotter-Green U. Cardiac resynchronisation therapy in patients with chronic heart failure. Heart 2015; 101: 1008-1014.
- Steinberg BA, Wehrenberg S, Jackson KP, Hayes DL, Varma N, Powell BD, Day JD, Frazier-Mills CG, Stein KM, Jones PW, Piccini JP. Atrioventricular and ventricular-to-ventricular programming in patients with cardiac resynchronization therapy: results from ALTITUDE. J Interv Card Electrophysiol 2015; 44: 279-287.
- Sade LE, Demir O, Atar I, Muderrisoglu H, Ozin B. Effect of right ventricular pacing lead on left ventricular dyssynchrony in patients receiving cardiac resynchronization therapy. Am J Cardiol 2009; 103: 695-700.
- Guo T, Li R, Zhang L, Luo Z, Zhao L, Yang J, Pu L, Hua B. Biventricular pacing with ventricular fusion by intrinsic activation in cardiac resynchronization therapy. Int Heart J 2015; 56: 293-297.
- Tayal B, Gorcsan J, Delgado-Montero A, Goda A, Ryo K, Saba S, Risum N, Sogaard P. Comparative long-term outcomes after cardiac resynchronization therapy in right ventricular paced patients versus native wide left bundle branch block patients. Heart Rhythm 2016; 13: 511-518.
- Pu LJ, Wang Y, Zhao L, Zhao L, Luo ZL, Hua BT, Han MH, Li SM, Yang J, Li L, Peng YZ, Guo T. Cardiac Resynchronization Therapy (CRT) with right ventricular sense triggered left ventricular pacing benefits for the hemodynamics compared with standard CRT for chronic congestive heart failure: a cross-over study. Cardiol J 2015; 22: 80-86.
- Gopi A, Sundar G, Yelagudri S, Lalukota K, Sridevi C, Narasimhan C. Atrial synchronous left ventricular only pacing with VDD pacemaker system-a cost effective alternative to conventional cardiac resynchronization therapy. Indian Heart J 2014; 66: 612-616.
- Antonini L, Auriti A, Pasceri V, Meo A, Pristipino C, Varveri A, Greco S, Santini M. Optimization of the atrioventricular delay in sequential and biventricular pacing: physiological bases, critical review, and new purpose. Europace 2012; 14: 929-938.
- Starling RC, Krum H, Bril S, Tsintzos SI, Rogers T, Hudnall JH, Martin DO. Impact of a novel adaptive optimization algorithm on 30-day readmissions: evidence from the adaptive CRT Trial. JACC Heart Fail 2015; 3: 565-572.
- Lim S. Ventricular safety pacing, ventricular sense response and ventricular tachycardia. Heart Rhythm 2010; 7: 567-569.
- Rafie R, Qamruddin S, Ozhand A, Taha N, Naqvi TZ. Shortening of atrioventricular delay at increased atrial paced heart rates improves diastolic filling and functional class in patients with biventricular pacing. Cardiovasc Ultrasound 2012; 10: 2.
- Sun JP, Lee AP, Grimm RA, Hung MJ, Yang XS, Delurgio D, Leon AR, Merlino JD, Yu CM. Optimisation of atrioventricular delay during exercise improves cardiac output in patients stabilised with cardiac resynchronisation therapy. Heart 2012; 98: 54-59.
- Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013; 159: 1070-1118.
- Kazemisaeid A, Rezvanfard M, Sadeghian H, Lotfi Tokaldany M, Mardanloo AS, Fathollahi MS. Comparison between Tissue Doppler Imaging (TDI) and Tissue Synchronization Imaging (TSI) in evaluation of left ventricular dyssynchrony in patients with advanced heart failure. Echocardiography 2012; 29: 7-12.
- Dandrea A, Caso P, Cuomo S, Scarafile R, Salerno G, Limongelli G, Di Salvo G, Severino S, Ascione L, Calabro P, Romano M, Romano G, Santangelo L, Maiello C, Cotrufo M, Calabrò R. Effect of dynamic myocardial dyssynchrony on mitral regurgitation during supine bicycle exercise stress echocardiography in patients with idiopathic dilated cardiomyopathy andnarrow QRS. Eur Heart J 2007; 28: 1004-1011.
- Cobb DB, Gold MR. The role of atrioventricular and interventricular optimization for cardiac resynchronization therapy. Card Electrophysiol Clin 2015; 7: 765-779.
- Khaykin Y, Exner D, Birnie D, Sapp J, Aggarwal S, Sambelashvili A. Adjusting the timing of left-ventricular pacing using electrocardiogram and device electrograms. Europace 2011; 13: 1464-1470.
- Boriani G, Gardini B, Diemberger I, Bacchi Reggiani ML, Biffi M, Martignani C, Ziacchi M, Valzania C, Gasparini M, Padeletti L, Branzi A. Meta-analysis of randomized controlled trials evaluating left ventricular vs. biventricular pacing in heart failure: effect on all-cause mortality and hospitalizations. Eur J Heart Fail 2012; 14: 652-660.
- Gasparini M, Galimberti P, Ceriotti C. The importance of increased percentage of biventricular pacing to improve clinical outcomes in patients receiving cardiac resynchronization therapy. Curr Opin Cardiol 2013; 28: 50-54.