ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 13
Extracts of Potentilla discolor bunge protect islet β-cells against streptozotocin induced injury
Xing Xu1, Yudong Zheng1, Lian Liu1, Wei Huang2 and Jiangrong Huang1*
1Medical School of Yangtze University, Jingzhou, PR China
2Endocrine Department, the Third Clinical Medical School, Yangtze University, Jingzhou, PR China
- *Corresponding Author:
- Jiangrong Huang
Medical School of Yangtze University
Hubei Province, PR China
Accepted date: May 9, 2017
Aim: To investigate whether Potentilla discolor Bunge (PDB) extracts could protect islet β cells and promote cell proliferation and insulin secretion.
Methods: β-cell injury model was induced by Streptozotocin. Cell viability, insulin secretion and gene expression were measured to investigate the protective effects of PDB extract on RIN-m5f β cells damaged by streptozotocin.
Results: PDB extracts inhibited cell damage, improved cell viability, promoted insulin secretion, and increased protein level of p-Fox O1 in RIN-m5f β cells in a dose-dependent manner.
Conclusions: PDB extracts exhibit protective role in streptozotocin induced β cell injury model. The mechanism may be related to increased phosphorylation of Fox O1, which increases the number and improves the function of β cells.
Keywords
EPD, RIN-m5f, Protective effect, Fox O1
Introduction
Diabetes Mellitus (DM) is a metabolic disorder characterized by systemic control of glucose dysfunction and insulin secretion imbalance. The prevalence of diabetes in Chinese adults has risen to 11.6%, accounting for 1/3 of the number of patients worldwide [1]. According to data released by WHO, diabetes has become the third major disease that threatens human health following cancer and cardiovascular diseases. Islet β cells are the only cells that secrete insulin, the maintenance of normal amount and function of insulin is necessary for the body to secret adequate insulin to maintain the balance of the body. Streptozotocin (STZ) is a compound derived from Streptomyces achromogenes that has been used for the treatment of pancreatic β cell carcinoma. Due to its toxicity to islet β cells, STZ has been used to induce cell or animal model of diabetes [2].
Forkhead box O (FoxO) is a transcription factor that plays an important role in the differentiation, proliferation and survival of various cells such as adipocytes, hepatocytes, and islet β cells. FoxO1 regulates the number of and function of β cells, and plays an important role in the development of diabetes [3,4].
Potentilla discolor Bunge (PDB) is a Rosaceae plant with various effects such as clearing heat and detoxicating, curing dysentery to arrest hematochezia. In diabetic rat model, PDB not only significantly reduced blood glucose but also promoted the repair of β cells and the release of insulin [5]. This study aimed to investigate whether PDB extracts could protect islet β cells and promote cell proliferation and insulin secretion. We established STZ induced damage to RIN-m5f cells as the model and examined the effects of PDB extracts.
Materials and Methods
Reagents
Potentilla discolor was identified by Shihua Wang, associate chief pharmacist at Department of Pharmacy at Jingzhou Chinese Medicine Hospital. PDB extracts were prepared as described previously [5]. Roswell Park Memorial Institute (RPMI) 1640 medium, penicillin and streptomycin were from Genom Biopharmaceutical Company. Fetal bovine serum was from HyClone Company. Thiazole blue (3-(4, 5- dimethylthiazol-2-yl)-2, 5-diphenyltetrazoliumbromide, MTT) was from AMRESCO Company. Streptozocin (STZ) was from Sigma. Rat insulin ELISA kit was from Mercodia (Sweden). Fox O1 monoclonal antibody, p-Fox O1 polyclonal antibody and β-actin antibody were from Abcam.
Cell culture
Rat RIN-m5f β cells were provided by Collection Center at Wuhan University and cultured in RPMI1640 medium supplemented with 10% fetal bovine serum, penicillin (100 U/ml) and streptomycin (100 mg/ml) at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. The medium was changed every two days. Cells were used when they were at the logarithmic phase of growth.
MTT assay
Cells were seeded into 96-well culture plates and cultured in a humidified chamber at 37°C overnight. Next the cells were treated with 0.25, 0.5, or 1 mg/ml PDB extracts for 48 h. Each day for three consecutive days, viable cells were evaluated with MTT assay kit (Sigma, USA) according to the manufacturer’s instructions. 20 μL MTT (5 mg/ml) solution was added to each well in 96-well plates and the plates were incubated at 37°C for 4 h, then 150 μL dimethyl sulfoxide (DMSO) was added to each well in 96-well plates and the plates were incubated at room temperature for 10 min. The absorption value of every well (A) was read at 492 nm using a microplate reader (ELX800, Bio-Tek, USA).
ELISA
Cells were treated with 0.25, 0.5, or 1 mg/ml PDB extracts for 48 h. The culture medium was collected and insulin content was determined by using ELISA kit according to the manufacturer’s instructions.
Western blot analysis
Cells were treated with 0.25, 0.5, or 1 mg/ml PDB extracts. The cells were collected and total protein was isolated from the cells and quantitated by BSA method. 30 μg protein was loaded onto a 10% Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferred to Polyvinylidene Difluoride (PVDF) membrane. Next, the membrane was incubated with specific antibody for FoxO1, p- FoxO1 or β-actin at 4ºC overnight, followed by incubation with secondary antibody for 1 h at room temperature. The membrane was developed using ECL kit (Pierce, Rockford, IL, USA) and exposed to X-ray film. Bands on the films were analysed by Image.plus5.1.
Statistical analysis
The data were expressed as ͞x ± s and analysed by SPSS 17.0 software. ANOVA was used to compare the differences among the groups. P<0.05 indicated that the difference was statistically significant.
Results
PDB extracts promotes the survival of RIN-m5f cells after STZ injury
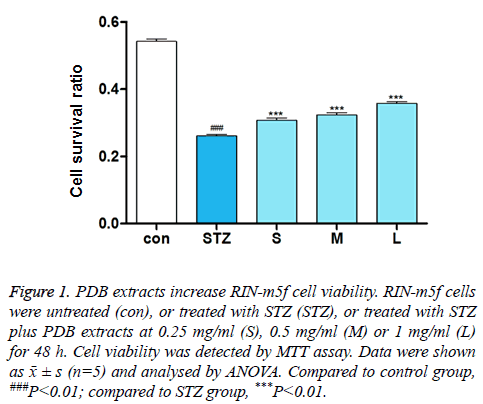
As shown in Figure 1, the number of cells in STZ injury group was significantly lower than that in the control group (P<0.01). Compared with the injury group, 0.25, 0.5 and 1 mg/ml PDB extracts could increase the number of cells in a dose-dependent manner and the differences were significant (P<0.01). These results suggest that PDB extracts effectively repair STZ induced β-cell injury.
Figure 1: PDB extracts increase RIN-m5f cell viability. RIN-m5f cells were untreated (con), or treated with STZ (STZ), or treated with STZ plus PDB extracts at 0.25 mg/ml (S), 0.5 mg/ml (M) or 1 mg/ml (L) for 48 h. Cell viability was detected by MTT assay. Data were shown as ͞x ± s (n=5) and analysed by ANOVA. Compared to control group, ###P<0.01; compared to STZ group, ***P<0.01.
PDB extracts promotes the secretion of insulin by RIN-m5f cells
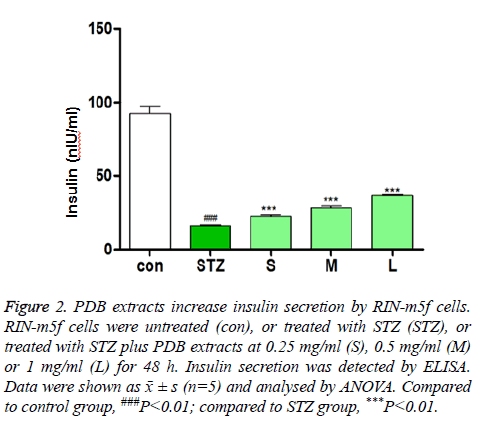
Insulin secretion of RIN-m5f cells was determined by ELISA. As shown in Figure 2, after 48 h treatment with PDB extracts, insulin secretion in the injured group significantly decreased compared to control group (P<0.01). Compared to injury group, insulin secretion increased in cells treated by PDB extracts in a dose-dependent manner and the differences were significant (P<0.01). These results suggest that PDB extracts effectively promote STZ injured β-cells to secret insulin.
Figure 2: PDB extracts increase insulin secretion by RIN-m5f cells. RIN-m5f cells were untreated (con), or treated with STZ (STZ), or treated with STZ plus PDB extracts at 0.25 mg/ml (S), 0.5 mg/ml (M) or 1 mg/ml (L) for 48 h. Insulin secretion was detected by ELISA. Data were shown as ͞x ± s (n=5) and analysed by ANOVA. Compared to control group, ###P<0.01; compared to STZ group, ***P<0.01.
PDB extracts promotes the phosphorylation of FoxO1 in RIN-m5f cells
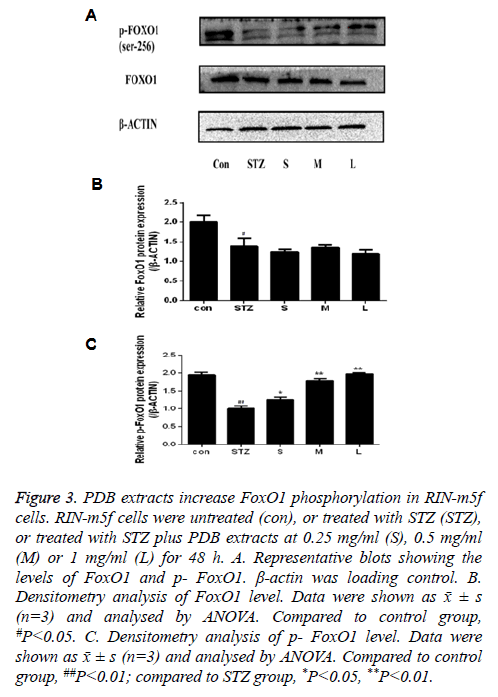
Compared to control group, FoxO1 and p-FoxO1 protein level in injured group decreased (P<0.05). Compared to injury group, FoxO1 protein level did not change significantly but p- FoxO1 protein level increased significantly in cells treated by PDB extracts in a dose-dependent manner and the differences were significant (P<0.01) (Figure 3). These results suggest that PDB extracts effectively promote FoxO1 phosphorylation in STZ injured β-cells.
Figure 3: PDB extracts increase FoxO1 phosphorylation in RIN-m5f cells. RIN-m5f cells were untreated (con), or treated with STZ (STZ), or treated with STZ plus PDB extracts at 0.25 mg/ml (S), 0.5 mg/ml (M) or 1 mg/ml (L) for 48 h. A. Representative blots showing the levels of FoxO1 and p- FoxO1. β-actin was loading control. B. Densitometry analysis of FoxO1 level. Data were shown as ͞x ± s (n=3) and analysed by ANOVA. Compared to control group, #P<0.05. C. Densitometry analysis of p- FoxO1 level. Data were shown as ͞x ± s (n=3) and analysed by ANOVA. Compared to control group, ##P<0.01; compared to STZ group, *P<0.05, **P<0.01.
Discussion
The damage model of islet cells is mostly induced by high glucose stimulation or STZ treatment [6-9]. The action time of STZ is shorter than high glucose stimulation, and the damage is obvious. Therefore, this study used STZ (4 mmol/L) to induce RIN-m5f β-cell injury model. Maintaining the number of β cells and protecting β cell function is the key to prevent the occurrence and deterioration of type 2 diabetes [10,11]. In this study, we found that PDB extracts could effectively prevent the damage of STZ to the survival and insulin secretion of RIN-m5f β-cells in a dose-dependent manner.
PI3K/AKT signaling pathway is involved in the regulation of the number and function of β cells [12,13]. PI3K/AKT signaling promotes Fox O1 phosphorylation, and phosphorylated Fox O1 could induce β-cell differentiation and insulin secretion, and protected the number and function of β cells [14]. In this study, we found that the expression of FoxO1 and p-FoxO1 in STZ injured β cells decreased, but PDB extracts increased p-FoxO1 level in β-cells in a dose-dependent manner. Therefore, PDB extracts may promote β cell differentiation and insulin secretion by affecting the phosphorylation of Fox O1.
In conclusion, this study demonstrated that PDB extracts exhibit protective effect on STZ damaged RIN-m5f cells, they increase the number of β cells and promote the release of insulin and the phosphorylation of Fox O1. However, the potential efficacy and possible side effects of PDB in diabetes patients remain unclear. Further clinical trials are needed to confirm the utilization of PDB extracts for the prevention and treatment of diabetes.
Acknowledgement
The study was supported by Hubei Province health and family planning scientific research project (WJ2016-YZ-07) and Hubei Province Health Department young talents project (QJX2012-20).
Conflict of Interest
No
References
- Xu Y, Wang L, He J, Bi Y, Li M. Prevalence and control of diabetes in Chinese adults. JAMA 2013; 310: 948-959.
- Palm F, Ortsater H, Hansell P, Liss P, Carlsson PO. Differentiating between effects of streptozotocin per se and subsequent hyperglycemia on renal function and metabolism in the streptozotocin-diabetic rat model. Diabetes Metab Res Rev 2004; 20: 452-459.
- Kikuchi O, Kobayashi M, Amano K. FoxO1 gain of function in the pancreas causes glucose intolerance, polycystic pancreas, and islet hypervascularization. PLoS One 2012; 7: 232-249.
- Kobayashi M, Kikuchi O, Sasaki T, Kim HJ, Yokota-Hashimoto H. FoxO1 as a double-edged sword in the pancreas: analysis of pancreas- and β-cell-specific FoxO1 knockout mice. Am J Physiol Endocrinol Metab 2012; 302: 603-613.
- Zhang L, Yang J, Chen XQ, Zan K, Wen XD. Antidiabetic and antioxidant effects of extracts from Potentilla discolor Bunge on diabetic rats induced by high fat diet and streptozotocin. J Ethnopharmacol 2010; 132: 518-524.
- Jeng JY, Yeh TS, Chiu YH. Linoleic acid promotes mitochondrial biogenesisand maintains mitochondrial structure for prevention of streptozotocin damage in rin-m5f cells. Biosci Biotechnol Biochem 2009; 73: 1262-1267.
- Hu CM, Li JS, Cheah KP, Lin CW, Yu WY. Effect of Sanguis draconis (a dragons blood resin) on streptozotocin- and cytokine-induced β-cell damage, in vitro and in vivo. Diabetes Res Clin Pract 2011; 94: 417-425.
- Onoue S, Hanato J, Yamada S. Pituitary adenylate cyclase-activating polypeptide attenuates streptozotocin-induced apoptotic death of RIN-m5F cells through regulation of Bcl-2 family protein mRNA expression. FEBS J 2008; 275: 5542-5551.
- Fu YY, Kanq KJ, Ahn JM. Hyperbilirubinemia reduces the streptozotocin-induced pancreatic damage through attenuating the oxidative stress in the Gunn rat. Tohoku J Exp Med 2010; 222: 265-273.
- Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science 2005; 307: 380-384.
- Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58: 773-795.
- Wang C, Chen X, Ding X, He Y, Gu C. Exendin-4 promotes beta cell proliferation via pi3k/AKT signalling pathway. Cell Physiol Biochem 2015; 35: 2223-2232.
- Hughes E, Huang C. Participation of Akt, menin, and p21 in pregnancy-induced beta-cell proliferation. Endocrinology 2011; 152: 847-855.
- Shao S, Nie M, Chen C, Chen X, Zhang M. Protective action of liraglutide in beta cells under lipotoxic stress via PI3K/Akt/FoxO1 pathway. J Cell Biochem 2014; 115: 1166-1175.