ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 19
Ephedra-cinnamomi attenuates cerebral ischemia-induced memory deficits via TLR4/MyD88/p38 MAPK pathway
Liang-Kui Xu1, Xiao-Ming Wang1, Xiao-Mei Tan1,2, Xue-Feng Xing1,2, Qing-Fa Tang1,2 and Jia-Bo Luo1,2*
1School of Traditional Chinese Medicine, Southern Medical University, Guangzhou, PR China
2Guangdong Provincial Key Laboratory of Chinese Medicine Pharmaceutics, Southern Medical University, Guangzhou, PR China
- *Corresponding Author:
- Jia-Bo Luo
School of Traditional Chinese Medical Sciences
Southern Medical University, PR China
Accepted on September 18, 2017
Background: Cerebral ischemia is a common and serious neurological disease. Ephedra-cinnamomi composed of Ephedra and cinnamomi is a famous herb-pair in Traditional Chinese Medicine. The aim of present study was to investigate the effect of Ephedra-cinnamomi on cerebral ischemia injury in rats.
Material and Methods: Four-Vessel Occlusion (4-VO) method was applied to induce global cerebral ischemia injury. Rats were treated with Ephedra-cinnamomi (3, 6 and 12 g/kg, i.g.) while the untreated rates were regarded as the negative control. Then memory capacity was measured by Step through Passive Avoidance (STPA) test and Morris Water Maze (MWM) test. Pro-inflammatory cytokines levels were detected by Enzyme Linked Immunosorbent Assay (ELISA). Additionally, the protein expression levels of Toll-Like Receptor (TLR) 4, myeloid differentiation factor 88 (MyD88) and phosphorylated p38 mitogen-activated protein kinases (p-p38MAPKs) were detected by Western blot.
Results: The results revealed that Ephedra-cinnamomi attenuated the cerebral ischemia-induced memory deficits and ameliorated histopathological changes. In addition, Ephedra-cinnamomi was able to reduce the pro-inflammatory cytokines production and inhibit the activation of TLR4/MyD88/p38 MAPK pathway in 4-VO induced rats model.
Conclusion: The results indicated that Ephedra-cinnamomi significantly ameliorates the ischemic brain injury in rats. Furthermore, the molecular mechanism of improved ischemic brain by Ephedracinnamomi is associated with the inhibition of TLR4/p38 MAPK pathway and the suppression of proinflammatory cytokines production.
Keywords
Ephedra-cinnamomi, Cerebral ischemia, Inflammatory cytokines, TLR4, p38 MAPK.
Introduction
Cerebral ischemia is one of the most leading causes of death and permanent disability worldwide [1]. Ischemic brain injury is triggered by a series of complicated biochemical and molecular mechanisms which initiates the ischemic cascade including excitotoxic glutamatergic signaling, ionic imbalance, free-radical reactions and inflammation [2-4]. Also, aberrant activation of inflammation pathways and significant increased secretion of pro-inflammatory cytokines [5,6] has been observed in cerebral ischemia. Toll-Like Receptor (TLR) 4, a member of TLRs family, promotes the activation of Mitogen- Activated Protein Kinases (MAPKs) and finally results in the overproduction of pro-inflammatory cytokines including Tumor Necrosis Factor-α (TNF-α), Interleukins (IL) IL-6 and IL-1β. Recently, growing evidences have indicated that activation of TLR4/MAPK pathway contributes to the development of cerebral ischemia [7,8] suggesting that TLR4/ MAPK pathway may be an effective therapeutic target of cerebral ischemia.
Ephedra-cinnamomi is composed of ephedra (stems of Ephedra sinica) and cinnamomi (twig of Cinnamomum cassia). Clinically, Ephedra-cinnamomi was applied to treat common cold, rheumatic diseases and cough. Furthermore, components of ephedra have the ability to accelerate motor function recovery in rats after cerebral ischemia and promote neural remodeling [9]. Recently, cinnamaldehyde, the major constituent of cinnamomi, has been proved to possess antineuroinflammatory activity [10]. The cinnamaldehyde could effectively ameliorate neurological deficit scores, brain edema and infarct volume in Permanent Middle Cerebral Artery Occlusion (PMCAO)-induced cerebral ischemia in rats [11]. However, the effects of the prescription of ephedra-cinnamomi on the cerebral ischemia are still unknown.
Therefore, this study was to investigate the pharmacological characteristics of ephedra-cinnamomi on cerebral ischemia. A rat model of cerebral ischemia was established with the four- Vessel Occlusion (4-VO). We firstly assessed the effects of ephedra-cinnamomi on memory behaviors by behavioral assessments including STPA and MWM. Then the influences of ephedra-cinnamomi on histopathological changes in 4-VOinduced were observed by hematoxylin and eosin (H and E) stain assay. To explore the role of ephedra-cinnamomi in inflammatory responses in cerebral ischemia, the levels of proinflammatory factor secretion and expression levels of TLR4/ MAPK signaling pathway was measured in 4-VO induced rats’ model.
Materials and Methods
Animals
A total of fifty male Sprague-Dawley rats (180 ± 20 g) were purchased from the Laboratory Animal Center of the Southern Medical University (Guangzhou, China) and the rates were housed in 23 ± 1°C with 45% humidity. All rates were housed in a 12 h light/dark cycle with access to water and food freely for at least 5 d before the experiment. The experiments were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996).
Drugs and administration
Traditional Chinese medicines ephedra (No. 110901) and cinnamomi (No. 121001) were purchased from Guangzhou Zhixin Pharmaceutical Co., Ltd (Guangzhou, China). Both medicines were identified by Prof. Ji Ma (School of Chinese Medicine, Southern Medical University, China) and the preparation of ephedra-cinnamomi (ephedra/cinnamomi: 3:2) was based on our previous study [12]. The ephedra-cinnamomi was diluted to 3, 6 and 12 g/kg (irrigation, i.g.) using 0.9% saline sodium chloride. Then, ephedra-cinnamomi (2 ml/kg) was administrated to rats by gavage after the day of the exposure to the surgery of cerebral ischemia once from day 2 to 16 and the drug was administrated 1 h before the behavioral test. Normal saline was administrated to rats in negative control groups.
Surgery of cerebral ischemia (d 1)
Rats model of transient global cerebral ischemia was estimated through 4-VO procedures as previously described [13]. Briefly, after anesthetization with 10% chloral hydrate (350 mg/kg, i.g.), common carotid arteries of rats were exposed ventrally and vertebral arteries were permanently electrocauterized. Then, rates were routinely allowed to recover from anesthesia for 24 h. Ischemia was induced by occluding both carotid arteries using non-traumatic aneurysm clips. The clips were removed 10 min after occlusion to restore blood flow and arterial patency. Meanwhile, the rectal temperature was continuously monitored to prevent hypothermia during anesthesia and surgery. The same surgical operation was performed on sham-operated animals without carotid ligation.
Step-through passive avoidance test (d 8 to 9)
Memory evaluation was performed using a step-through passive avoidance apparatus (Bio Medica, Ltd, Osaka, Japan) [13]. The apparatus contained a chamber with two compartments (dark/illuminated), which was divided by a sliding door. The darkroom has a stainless steel mesh floor for electric footrest. During training, rats were kept in the illuminated compartment separately for 1 min and then raised the door. Once the rats entered the dark compartment, the guillotine door was closed and the electric foot shock was performed to rats (0.5 mA, 3 s). The latency to enter the dark compartment was recorded. Also, the test was performed after 24 h training, the incubation period was recorded using the same program, but no foot shock was delivered. If rats don’t enter the dark compartment within 180 s, the rats will be returned to the home cage and the ceiling score was recorded as 180 s.
Morris water maze test (d 11 to 16)
To further assess whether the ephedra-cinnamomi and the exposure to surgery of cerebral ischemia affect the spatial learning and memory in rats, the Morris water maze test was conducted. The circular pool (120 cm diameter × 60 cm height) filled with water (22°C; 38 cm deep) was used. A transparent circular platform (10 cm in diameter) was set at the east quadrant, which was 40 cm near the wall and was inundated 2 cm below the water level. The rats were trained twice daily for 5 successive days with an intermission of 3 h between test. The computer-controlled tracking system was employed to measure the swimming distance, the latency to reach the platform and the swimming velocity. Rats were leaded to the platform by person so that the rats can’t find the platform in 90 s. On the16th d (i.e., 24 h after the last acquisition test), the platform was removed and the spatial tracking test was performed. It allows 90 s for rats to reach the target quadrant and swimming distance. After the spatial tracking test, the visible platform trials were carried out for 3 consecutive days. In this section, the visible platform is located at 1 cm above the surface of the water and rats were permitted to find the target three times daily. The result was recorded and analysed.
Enzyme-linked immunosorbent assay (ELISA)
After the behavioral test, the rats were ethically decapitated. Then the levels of TNF-α, IL-6 and IL-1β in the prefrontal cortex and hippocampus were measured by ELISA. The prefrontal cortex and hippocampus sample was prepared with slightly modified based on the previous study [14]. TNF-α, IL-6 and IL-1β levels in prefrontal cortex and hippocampus were assayed with ELISA kits (Raybiotech, USA) respectively. Then, the Optical Density (OD) values at 450 nm wavelength were detected for statistical analysis.
Hematoxylin–eosin staining analysis
24 h after the treatment of ephedra-cinnamomi, the rats were anaesthetized with 10% chloral hydrate and then perfused with physiological saline. The brain tissues were removed and immediately fixed at 4°C in formaldehyde solution overnight. After dehydrated and embedded with paraffin, the specimens were cut into 5 μm thick coronal sections. Then, the sections were deparaffinized, rehydrated, and re-stained with Hematoxylin and Eosin (H and E), respectively. Finally, light microscope (Olympus, Tokyo, Japan) examination was performed to observe the histopathological changes of the cortex in different groups.
Western blot assay
Total protein extraction of rat prefrontal cortex and hippocampus was prepared with a standard method. Then proteins (2 μg) were resolved by 8% SDS-PAGE, and then electrophoretically transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). Proteinsblots were incubated with primary antibody for 1h and secondary antibodies for 45 min at 4°C. Western blot bands were visualized via enhanced chemiluminescence (Cell Signaling Technology).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA). All data were presented as the means ± S.E.M. Repeated measures ANOVA was used to analyse the changes of body weights, speeds and escape latencies in the MWM test. Other data were analysed through one-way Analysis of Variance (ANOVA) followed by Bonferroni's multiple comparison tests. For all results, p<0.05 was defined as significant differences.
Results
Attenuation of cerebral ischemia-induced memory impairment by ephedra-cinnamomi in the passive avoidance test
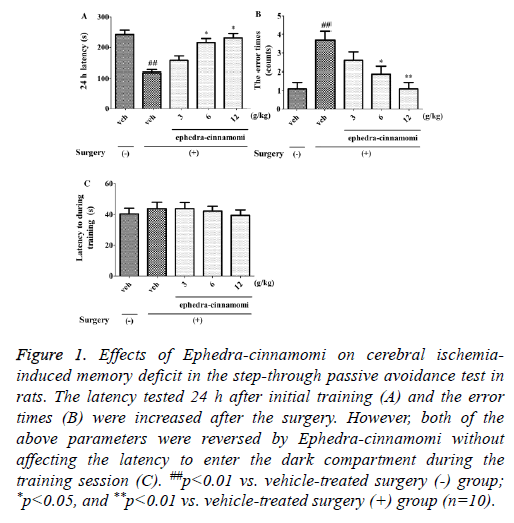
As shown in Figure 1, the latency tested at 24 h after initial training (One-way ANOVA, p<0.01; Figure 1A) was decreased and the error time (One-way ANOVA, p<0.01; Figure 1B) was increased after the surgery. However, both of the above parameters were reversed by ephedra-cinnamomi (6 and 12 g/kg, i.g.) without affecting the latency during the training session (p>0.05; Figure 1C). These results suggested that ephedra-cinnamomi improved cerebral ischemia-caused memory impairment in the passive avoidance test.
Figure 1: Effects of Ephedra-cinnamomi on cerebral ischemiainduced memory deficit in the step-through passive avoidance test in rats. The latency tested 24 h after initial training (A) and the error times (B) were increased after the surgery. However, both of the above parameters were reversed by Ephedra-cinnamomi without affecting the latency to enter the dark compartment during the training session (C). ##p<0.01 vs. vehicle-treated surgery (-) group; *p<0.05, and **p<0.01 vs. vehicle-treated surgery (+) group (n=10).
Improvement of memory impairment by ephedracinnamomi in the Morris water maze test
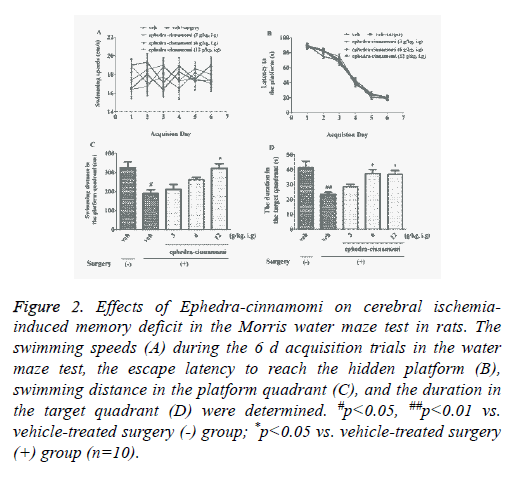
To assess the influence of ephedra-cinnamomi on cerebral ischemia-induced spatial memory deficits, the Morris water maze test was performed. Figure 2 showed that no significant differences was observed in swimming velocity (p>0.05; Figure 2A). In addition, acquisition trial progressively reduced escape latency to reach the hidden platform (p>0.05; Figure 2B). It had been displayed that the surgery of cerebral ischemia decreased the swimming distance in platform quadrant (p<0.05; Figure 2C) 24 h after the last acquisition trial and reduced the duration in target quadrant (p<0.05; Figure 2D), respectively. However, both of the above parameters were reversed by ephedra-cinnamomi treatment (6 g/kg, i.g. for swimming distance; 6 and 12 g/kg, i.g. for duration). These results indicated that ephedra-cinnamomi ameliorated spatial memory deficit without affecting the local activity.
Figure 2: Effects of Ephedra-cinnamomi on cerebral ischemiainduced memory deficit in the Morris water maze test in rats. The swimming speeds (A) during the 6 d acquisition trials in the water maze test, the escape latency to reach the hidden platform (B), swimming distance in the platform quadrant (C), and the duration in the target quadrant (D) were determined. #p<0.05, ##p<0.01 vs. vehicle-treated surgery (-) group; *p<0.05 vs. vehicle-treated surgery (+) group (n=10).
Effects of Ephedra-cinnamomi on the histopathological changes in rats brain
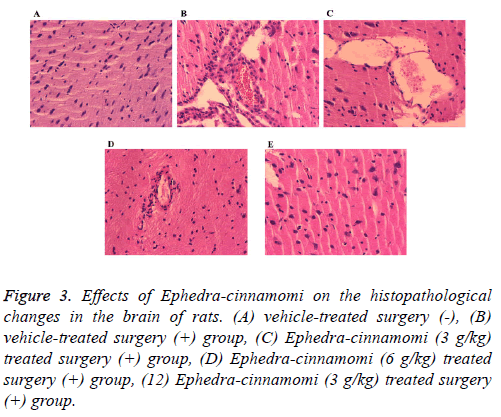
To observe the effects of Ephedra-cinnamomi on the histopathological changes in rats, HE staining has been performed. As shown in Figure 3, compared with the control group, ischemia surgery induced obvious infiltration of inflammatory cells in brain tissues of rats (Figure 3B). By contrast, Ephedra-cinnamomi treatment significantly alleviated brain injury (Figures 3C-3E).
Figure 3: Effects of Ephedra-cinnamomi on the histopathological changes in the brain of rats. (A) vehicle-treated surgery (-), (B) vehicle-treated surgery (+) group, (C) Ephedra-cinnamomi (3 g/kg) treated surgery (+) group, (D) Ephedra-cinnamomi (6 g/kg) treated surgery (+) group, (12) Ephedra-cinnamomi (3 g/kg) treated surgery (+) group.
Effects of Ephedra-cinnamomi on brain proinflammatory cytokines level in rats
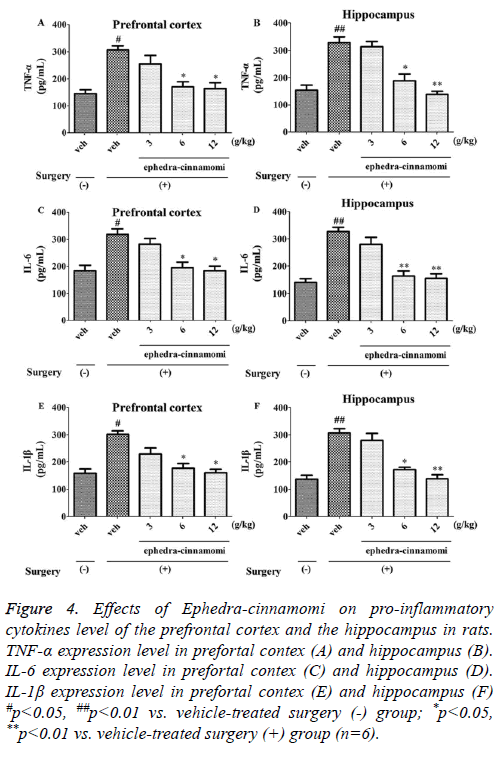
ELISA analysis (Figure 4) demonstrated that the ischemia surgery significantly increased the TNF-α levels (prefrontal cortex: p<0.05, Figure 4A; hippocampus: p<0.05; Figure 4B). However, both of the above parameters were reversed by ephedra-cinnamomi (6 and 12 g/kg: p<0.05.). Similar to TNF- α levels, the expression levels of IL-6 and IL-1β were significantly elevated after ischemia surgery (p<0.05; Figure 4A) and then the expression levels were greatly reduced with the treatment of ephedra-cinnamomi. These results indicated that Ephedra-cinnamomi ameliorated cerebral ischemiainduced memory impairment associated with the reversion of pro-inflammatory cytokines level in the prefrontal cortex and hippocampus.
Figure 4: Effects of Ephedra-cinnamomi on pro-inflammatory cytokines level of the prefrontal cortex and the hippocampus in rats. TNF-α expression level in prefortal contex (A) and hippocampus (B). IL-6 expression level in prefortal contex (C) and hippocampus (D). IL-1β expression level in prefortal contex (E) and hippocampus (F) #p<0.05, ##p<0.01 vs. vehicle-treated surgery (-) group; *p<0.05, **p<0.01 vs. vehicle-treated surgery (+) group (n=6).
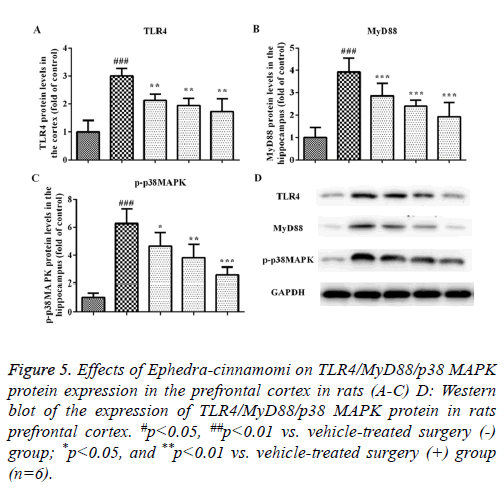
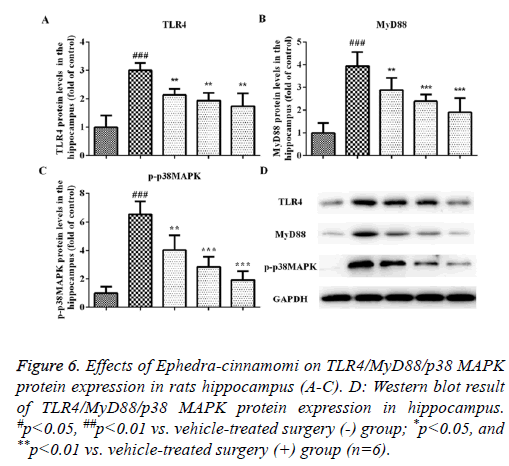
Effects of Ephedra-cinnamomi on the TLR4/MyD88 signaling pathway in rats
We measured the protein levels of TLR4, MyD88 and p-p38 MAPK by western blotting analysis. As illustrated in Figure 5, compared to the control group, the upregulation of TLR4 and MyD88 was observed in the model groups regarding the prefrontal cortex (TLR4: P<0.001, Figures 5A and 5D; MyD88: P<0.001, Figures 5B and 5D) and hippocampus (TLR4: P<0.001, Figures 6A and 6D; MyD88, P<0.001, Figures 6B and 6D). Accordingly, upregulation of p-p38 MAPK was observed in the prefrontal cortex (P<0.001, Figures 5C and 5D) and hippocampus (P<0.001, Figures 6C and 6D) of model groups. However, Ephedra-cinnamomi decreased the expression of TLR4, MyD88 and p38 MAPK model groups compared with the control groups (Figures 5 and 6). However, the phosphorylation levels of JNK and ERK were not affected by ephedra-cinnamomi (data not show).
Figure 5: Effects of Ephedra-cinnamomi on TLR4/MyD88/p38 MAPK protein expression in the prefrontal cortex in rats (A-C) D: Western blot of the expression of TLR4/MyD88/p38 MAPK protein in rats prefrontal cortex. #p<0.05, ##p<0.01 vs. vehicle-treated surgery (-) group; *p<0.05, and **p<0.01 vs. vehicle-treated surgery (+) group (n=6).
Figure 6: Effects of Ephedra-cinnamomi on TLR4/MyD88/p38 MAPK protein expression in rats hippocampus (A-C). D: Western blot result of TLR4/MyD88/p38 MAPK protein expression in hippocampus. #p<0.05, ##p<0.01 vs. vehicle-treated surgery (-) group; *p<0.05, and **p<0.01 vs. vehicle-treated surgery (+) group (n=6).
Discussion
From present study, Ephedra-cinnamomi could alleviate the cerebral ischemia-induced brain injury and memory deficits. Moreover, the upregulation of TNF-α, IL-6 and IL-1β both in the prefrontal cortex and hippocampus after the surgery was reversed by Ephedra-cinnamomi. Therefore, the underlying mechanism of the effects of ephedra-cinnamomi may be associated with the suppression of TLR4/MyD88/p38 MAPK pathway.
It has been well documented that 4-VO was widely used for cerebral ischemia model establishment. We found that Ephedra-cinnamomi ameliorated cerebral ischemia-induced memory impairment by decreasing the latency and the error times that were exposed to the surgery. Also, the latency to enter the dark compartment in rats model groups was not influenced by the activity of Ephedra-cinnamomi, indicating that memory behavioral deficits reversed by Ephedracinnamomi were not associated with affecting the locomotor activity in rats. The step-through passive avoidance test confirmed by Morris water maze test was proved by reversing swimming distance in the platform quadrant and the duration in the target quadrant by Ephedra-cinnamomi treatment. Although the activity of Ephedra-cinnamomi on cerebral ischemia is not fully understood, several reports have shown that ephedra/cinnamomi has the significant effects on cerebral ischemia/ischemia. For example, a previous study has demonstrated that cinnamomi may be beneficial to the treatment of brain ischemia-reperfusion injury partly due to its anti-inflammatory properties [15]. In addition, the behavior deficits that induced by cerebral ischemia were improved by ephedra involving neural remodeling [9]. The previous studies had been shown to be in line with our present study. Based on the data from the previous and present researches, it is suggested that ephedra-cinnamomi is effective in the treatment of cerebral ischemia.
Little is known about the possible mechanism of ephedracinnamomi in the treatment of cerebral ischemia. The inflammatory response was closely related to the pathophysiology of cerebral ischemia [16]. Also, the brain regions like prefrontal cortex and hippocampus, had been demonstrated to be closely associated with the cerebral ischemia [17,18]. Based on these evidences, the role of the inflammatory cytokine in the activity of Ephedra-cinnamomi in the prefrontal cortex and hippocampus was evaluated. The results showed TNF-α, IL-6 and IL-1β levels in the prefrontal cortex and hippocampus were elevated after exposure to the cerebral ischemia surgery, which is consistent with the previous studies [19,20]. However, the increase of inflammatory cytokines were markedly reversed by Ephedracinnamomi (6 and 12 g/kg, i.g.) in the brain regions, which suggested that the activity of Ephedra-cinnamomi (6 and 12 g/kg, i.g.) on the animal model of cerebral ischemia was associated with the decreased level of TNF-α, IL-6 as well as IL-1β. Consequently, the reduction of TNF-α, IL-6 and IL-1β in the brain with the treatment of ephedra-cinnamomi improved the outcome of behavioral deficits of the cerebral ischemia.
TLR-MyD88 association activates MAPK cascades, which further induces the expression of TNF-α, IL-6 and the maturation of IL-1β. P38 MAPK is activated by cellular stress including hypoxia and ischemia. The inhibition of p38 MAPK shows protective roles in the focal ischemic injury [21,22]. Moreover, the aberrant activation of TLR4/MyD88/p38 MAPK signaling has been observed in mice [23] and rats [21] with cerebral ischemia. In consistent with these studies, we found that the expression of TLR4, MyD88 and p-p38 MAPK were significantly elevated after cerebral ischemia surgery, which suggests the important roles of this signaling pathway in cerebral ischemia. Importantly, Ephedra-cinnamomi remarkably inhibited the elevated expression of TLR4, MyD88 and p-p38 MAPK. Therefore, it can be concluded that Ephedra-cinnamomi may inhibit the activation of TLR4/ MyD88/p38 MAPK in cerebral ischemic injury.
Conclusion
In conclusion, our results demonstrated that ephedracinnamomi improved the cerebral ischemia-induced memory behavioral deficits. The underlying mechanism was associated with the reduction of pro-inflammatory in the prefrontal cortex and hippocampus by inhibiting TLR4/MyD88/p38 MAPK pathway. The findings suggested that Ephedra-cinnamomi may be used as a potential neuroprotective drug for cerebral ischemia. However, further study is still needed involving the Ephedra-cinnamomi for cerebral ischemia treatment.
Acknowledgement
This study was supported by a grant from National Natural Science Foundation of China (No. 81030066).
Statement of Interest
All authors have declared that no competing interests exist in this project.
References
- Sveinsson OA, Kjartansson O, Valdimarsson EM. Cerebral ischemia/infarction-epidemiology, causes and symptoms. Laeknabladid 2014; 100: 271-279.
- Bae CY, Sun HS. TRPM7 in cerebral ischemia and potential target for drug development in stroke. Acta Pharmacol Sin 2011; 32: 725-733.
- Caldeira MV, Salazar IL, Curcio M, Canzoniero LM, Duarte CB. Role of the ubiquitin-proteasome system in brain ischemia: friend or foe? Prog Neurobiol 2014; 112: 50-69.
- de la Tremblaye PB, Raymond J, Milot MR, Merali Z, Plamondon H. Evidence of lasting dysregulation of neuroendocrine and HPA axis function following global cerebral ischemia in male rats and the effect of antalarmin on plasma corticosterone level. Horm Behav 2014; 65: 273-284.
- Bartl J, Link P, Schlosser C, Gerlach M, Schmitt A, Walitza S, Riederer P, Grunblatt E. Effects of methylphenidate: the cellular point of view. Atten Defic Hyperact Disord 2010; 2: 225-232.
- Fang L, Gao H, Zhang W, Zhang W, Wang Y. Resveratrol alleviates nerve injury after cerebral ischemia and reperfusion in mice by inhibiting inflammation and apoptosis. Int J Clin Exp Med 2015; 8: 3219-3226.
- Gao Y, Fang X, Tong Y, Liu Y, Zhang B. TLR4-mediated MyD88-dependent signaling pathway is activated by cerebral ischemia-reperfusion in cortex in mice. Biomed Pharmacother 2009; 63: 442-450.
- Trendelenburg G. Molecular regulation of cell fate in cerebral ischemia: role of the inflammasome and connected pathways. J Cereb Blood Flow Metab 2014; 34: 1857-1867.
- Chen Y, Xiao N, Lin L, Liu L. Ephedrine and naloxone promote nerve remodeling after cerebral ischemia. Zhongguo Zhong Yao Za Zhi 2009; 34: 1852-1856.
- Guo JY, Huo HR, Zhao BS, Liu HB, Li LF, Ma YY, Guo SY, Jiang TL. Cinnamaldehyde reduces IL-1beta-induced cyclooxygenase-2 activity in rat cerebral microvascular endothelial cells. Eur J Pharmacol 2006; 537: 174-180.
- Zhao J, Zhang X, Dong L, Wen Y, Zheng X, Zhang C, Chen R, Zhang Y, Li Y, He T. Cinnamaldehyde inhibits inflammation and brain damage in a mouse model of permanent cerebral ischemia. Br J Pharmacol 2015; 20: 5009-5029.
- Zheng FH, Wei P, Huo HL, Xing XF, Chen FL, Tan XM, Luo JB. Neuroprotective effect of Gui Zhi (Ramulus cinnamomi) on Ma Huang-(herb ephedra-) induced toxicity in rats treated with a ma Huang-Gui Zhi herb pair. Evid Based Complement Alternat Med 2015; 2005: 913461.
- Bin J, Wang Q, Zhuo YY, Xu JP, Zhang HT. Piperphentonamine (PPTA) attenuated cerebral ischemia-induced memory deficits via neuroprotection associated with anti-apoptotic activity. Metab Brain Dis 2012; 27: 495-505.
- An L, Li J, Yu ST, Xue R, Yu NJ, Chen HX, Zhang LM, Zhao N, Li YF, Zhang YZ. Effects of the total flavonoid extract of Xiaobuxin-Tang on depression-like behavior induced by lipopolysaccharide and proinflammatory cytokine levels in mice. J Ethnopharmacol 2015; 163: 83-87.
- Li TJ, Qiu Y, Mao JQ, Yang PY, Rui YC, Chen WS. Protective effects of Guizhi-Fuling-capsules on rat brain ischemia/reperfusion injury. J Pharmacol Sci 2007; 105: 34-40.
- Kim DW, Lee JC, Cho JH, Park JH, Ahn JH, Chen BH, Shin BN, Tae HJ, Seo JY, Cho JH. Neuroprotection of ischemic preconditioning is mediated by anti-inflammatory, not pro-inflammatory, cytokines in the gerbil hippocampus induced by a subsequent lethal transient cerebral ischemia. Neurochem Res 2015; 40: 1984-1995.
- Garcia-Chavez D, Gonzalez-Burgos I, Letechipia-Vallejo G, Lopez-Loeza E, Morali G, Cervantes M. Long-term evaluation of cytoarchitectonic characteristics of prefrontal cortex pyramidal neurons, following global cerebral ischemia and neuroprotective melatonin treatment, in rats. Neurosci Lett 2008; 448: 148-152.
- Yang Y, Li X, Zhang L, Liu L, Jing G, Cai H. Ginsenoside Rg1 suppressed inflammation and neuron apoptosis by activating PPARgamma/HO-1 in hippocampus in rat model of cerebral ischemia-reperfusion injury. Int J Clin Exp Pathol 2015; 8: 2484-2494.
- Wang YS, Li YX, Zhao P, Wang HB, Zhou R, Hao YJ, Wang J, Wang SJ, Du J. Anti-inflammation effects of oxysophoridine on cerebral ischemia-reperfusion injury in mice. Inflammation 2015; 38: 2259-2268.
- Zhu Y, Yang GY, Ahlemeyer B, Pang L, Che XM, Culmsee C, Klumpp S, Krieglstein J. Transforming growth factor-beta 1 increases bad phosphorylation and protects neurons against damage. J Neurosci 2002; 22: 3898-3909.
- Qiao H, Zhang X, Zhu C, Dong L, Wang L, Zhang X, Xing Y, Wang C, Ji Y, Cao X. Luteolin downregulates TLR4, TLR5, NF-kappaB and p-p38MAPK expression, upregulates the p-ERK expression, and protects rat brains against focal ischemia. Brain Res 2012; 1448: 71-81.
- Li H, Zhou S, Wu L, Liu K, Zhang Y, Ma G, Wang L. The role of p38MAPK signal pathway in the neuroprotective mechanism of limb postconditioning against rat cerebral ischemia/reperfusion injury. J Neurol Sci 2015; 357: 270-275.
- Wang L, Li Z, Zhang X, Wang S, Zhu C, Miao J, Chen L, Cui L, Qiao H. Protective effect of shikonin in experimental ischemic stroke: attenuated TLR4, p-p38MAPK, NF-kappaB, TNF-alpha and MMP-9 expression, up-regulated claudin-5 expression, ameliorated BBB permeability. Neurochem Res 2014; 39: 97-106.