ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 1
Dysadherin is highly expressed in ovarian cancer side population and plays important role in drug resistance of ovary cancer stem cells
Department of Obstetrics and GynaecologyWomen and Infants Hospital of Zhengzhou, Zhengzhou, Henan Province, PR China
- *Corresponding Author:
- Jun Cui
Department of Obstetrics and Gynaecology
Women and Infants Hospital of Zhengzhou
Henan
PR China
Accepted date: June 06, 2016
Purpose of investigation: Presence of subset of small population of cancer stem like cells called “Side population” (SP) cells, which are responsible for multi-drug resistance and cancer relapse. The present study was designed to reveal the possible link between elevated dysadherin and drug resistance of SP cells from high-grade ovarian cancer.
Materials and methods: The cancer samples were analysed for presence of SP cells by fluorescenceactivated cell sorting method (FACs). By siRNA technology, the expression of dysadherin in SP cells was compromised and therefore we compared the extent of drug and apoptosis sensitivity of SP cells.
Results: By FACS, we have identified 3.3% of side population cells in ovarian cancer. Further, the sorted SP cells showed enhanced expression of dysadherin, ABCG2 (ABC transporter), stem cell markers such as CD133, Oct-4, and EpCAM which are responsible for multi-drug resistance and maintenance of selfrenewal of SP cells. In addition, we showed that siRNA transfected SP cells, showed increased sensitivity towards different chemotherapeutic drugs and apoptosis.
Conclusion: Our data suggest that ovarian cancer SP cells possess elevated expression of dysadherin which are responsible for ABCG2 mediated drug resistance and reduced rate of apoptosis. Therefore, novel anticancer drugs targeting the expression of dysadherin could effectively prevent the tumour recurrence and metastasis.
Keywords
Apoptosis, Cancer stem cells, Dysadherin, Ovarian cancer, Side population
Introduction
Ovarian cancer is one of the leading causes for high mortality rate among other gynaecological malignancies responsible for approximately a 125,000 deaths annually [1,2]. Ovarian cancer is known to be the seventh most common cancer and the fifth leading cause of death among the women population, worldwide [3,4]. Although the fatality of ovarian cancer is not very severe in China (3.2 per 100,000 person/year), recent reports suggest an upward trend in the progression of this disease [5]. Although improvement has been made in the treatment of this disease, through the combination of surgery, radiation and chemotherapy using paclitaxel, gemcitabine, cisplatin, etc. but the overall five-year survival-rate is less than 40% [6], mainly because of chemotherapy failure and tumour recurrence [6,7]. Majority of the ovarian cancer are epithelial cells of origin and hence they are termed as epithelial ovarian cancer (EOC) [8]. Based on histological and pathological grades, the ovarian cancer is categorized as Type I (low-grade and heterogeneous) and Type II tumours (high-grade and mostly serous) [8,9]. The prevalence of type II high grade serous ovarian carcinoma (HGSC) is more than 80% which accounts for almost 90% of ovarian cancer deaths [10,11]. The current conventional treatment strategies are killing most of the neoplastic cells but they are failed to target the presence of small population of cancer stem cells or tumour-initiating cells (CSCs/TICs), which are responsible for chemotherapy resistance, tumour relapse and metastasis [11,12]. These characteristic features of CSCs are closely are associated with the properties of stem cells such as self-renewal, high differentiation potential, apoptosis resistance [13] and also possess intrinsic property of chemotherapy resistance [14]. Therefore, isolation and characterization of CSCs is an ultimate goal for the understanding of CSCs mediated ontogenesis and chemotherapy resistance.
The most common method used so far to identify the cancer stem cells are the FACs based Hoechst 33342 dye exclusion technique. During FACs analysis, a subset of cancer cells exclude the Hoechst 33342 dye and appear as a distinct population (SP) cells in dot plot analysis of the FACs are termed as side population (SP) cells. Studies in different cancers reported that SP cells shared all the remarkable features of CSCs includes rapid cell proliferation, tumour initiating capacity and expression of stemness genes [12,15,16]. Further, SP cells have enhanced expression of ATPase binding cassette (ABC) transporters such as ABCB1 (MDR1), ABCC1, ABCG2 (BCRP1) in SP cells plays a major role in multi-drug resistance [12,15,16]. In addition, accumulating experimental evidences showed that of dysadherin, a cell membrane glycoprotein plays important role in the progression and metastasis of several carcinomas, including the ovarian cancer and its expression was also found to be significantly elevated in the cancer stem cell population [17-23]. These interesting findings suggest over expression of dysadherin might be linked with and drug and apoptosis resistance of SP cells. Therefore, it is worth to understand the precise molecular mechanism of dysadherin regulated therapy failure and tumour recurrence in order to develop CSCs specific therapeutic agents. In the present study, we aimed to identify and characterize the SP cells from high grade serous ovarian carcinoma. Further, the FACs sorted SP cells were analysed for the functional link between aberrant expression of dysadherin and apoptosis and drug resistance in SP cells.
Materials and Methods
Sample collection
The cancer samples were collected from the patients at the time of surgery in Women & Infants Hospital of Zhengzhou, in accordance with the ethical rule approved by Women & Infants Hospital of Zhengzhou (ethical approval number is 2012734345). High grade ovarian cancer tissues were collected from the patients at the time of surgery by experienced pathologist in our hospital. Patients details: (n=18; age ranges from 39-47; tumour grade-T5 and T6; site-bilateral; FIGO stage-IIIC and IV). Cancer cells were then collected from the tumour samples and cultured for the required experiments.
Isolation and culture of cancer cells from the collected tumours
The collected tumour samples were mechanically dissociated and enzymatically digested in a 1:1 solution of Type III collagenase/hyaluronidase for 30 min at 37ºC and incubated at 37º C for 2 h in a shaking bath. In order to remove the clumps, sterile gauzes (pore diameter sizes: 200 mesh) were used, erythrolysis was performed in hypotonic solution (0.2% NaCl) and subsequently 1.2% NaCl was added to stop lysis. Cells were then cultured in DMEM with 10% FBS supplemented with antibiotics and maintained in T-75 flasks at 37º C in a humidified 5% CO2 & 95% air atmosphere. At ~ 90% confluency, cells were trypsinized into single cell suspension, using trypsin-EDTA, centrifuged at 5000 rpm at 6 minutes and the pellet was further resuspended in DMEM with 10% FBS.
FACS analysis - labelling with hoechst 33342
Study groups: Control-cells+Hoechst 33342 (n=7); Drug treated-cells+Verapamil+Hoechst 33342 (n=7). Cells in staining medium (app106 cells/ml of 10% DMEM) are labelled with Hoechst 33342 stock (sigma)-bis-benzimide (5 μl/ml) either with dye alone or in combination with drug (verapamil - 0.8 μl/ml). The cells were mixed and placed in water bath at 37ºC for 90 minutes exactly. After 90 minutes, cells were spin down (2000 rpm for 10 minutes at 4ºC) and resuspended in 500 μl of HBSS containing 10 mM HEPES. Finally, counter stained the cells with PI (propidium iodide) 2 μg/ml sample at 4ºC. Cells were filtered through a 50 μm nylon mesh (BD) to remove cell clumps into labelled FACS tubes. Separate tubes with medium (10% DMEM) were kept for sterile sorting of Side Population cells and main population cells. The cells were sorted using a flow cytometer (FACS Aria II; BD Biosciences; Franklin Lakes, NJ, USA). The Hoechst 33342 dye was excited at 355 nm and its dualwavelength fluorescence was analysed (blue-450 nm; red- 675 nm).
RT-PCR analysis
The RNA was isolated by using the RNA isolation kit (Invitrogen, Carlsbad, CA) and the RNA samples were treated with DNase I to exclude the DNA contamination. The following PCR primers were used for RT-PCR analysis. CD133: (F-TCT TGA CCG ACT GAG AC and R-ACT TGA TGG ATG CAC CAA GCA C); GAPDH: (F-TGG ACT CCA CGA CGT ACT CAG and R-ACA TGT TCC AAT ATG ATT CCA); ABCG2: (F-AGC TGC AAG GAA AGA TCC AA and R-TCC AGA CAC ACC ACG GAT AA); OCT-4: (F-ATC CTG GGG GTT CTA TTT GG and R-CTC CAG GTT GCC TCT CAC TC); EpCAM: (F-CTG CCA AAT GTT TGG TGA TG and R-ACG CGT TGT GAT CTC CTT CT); BCL-2: (FACA CTG TTA AGC ATG TGC CG and R-CCA GCT CAT CTC ACC TCA CA) [18,22,23]. GAPDH was used as an internal control. PCR parameters-95ºC for 2 min, 40 cycles of 95ºC for 30 sec, 55-60ºC for 1 min and 72º for 30-60 sec. PCR products were electrophoresed on a 1.2% agarose gel and stained with ethidium bromide. The band intensity was measured by using image-J. The values presented in the graph Figure 1C are the average values of 3 independent experiments.
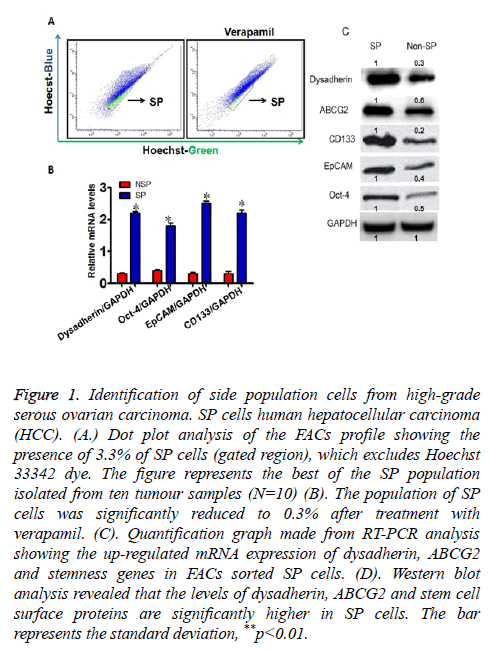
Figure 1: Identification of side population cells from high-grade serous ovarian carcinoma. SP cells human hepatocellular carcinoma (HCC). (A.) Dot plot analysis of the FACs profile showing the presence of 3.3% of SP cells (gated region), which excludes Hoechst 33342 dye. The figure represents the best of the SP population isolated from ten tumour samples (N=10) (B). The population of SP cells was significantly reduced to 0.3% after treatment with verapamil. (C). Quantification graph made from RT-PCR analysis showing the up-regulated mRNA expression of dysadherin, ABCG2 and stemness genes in FACs sorted SP cells. (D). Western blot analysis revealed that the levels of dysadherin, ABCG2 and stem cell surface proteins are significantly higher in SP cells. The bar represents the standard deviation, **p<0.01.
RNA interference
The small interfering RNA (siRNA) specific to dysadherin (Gene Bank accession No. AB072911) [22,23], were purchased from Dharmacon (Lafayette, CO 80026). SiRNA Transfection (final concentration of 200 nm) was performed as per the Dharmacom protocol. The transfected SiRNA cells were analysed after 48 hours.
Cell resistance assay
The obtained SP and non-SP cells were cultured in 96-well plates at a concentration of 1 × 103 cells/plate. After 24 hours, 5-fluorouracil (5-FU) was added to all cultures to a final concentration of 10 μg/ml. Similarly, cells were also treated with Cisplatin (20 μmol/L), Paclitaxel (2 μmol/L). The plates were placed in a hatch box for 48 h. Then each well was supplemented with CCK-8 (10 μl) solution and the plates were incubated for 3 hours. The mean value of OD450 obtained was represented as a graph. Cell resistance in both groups was calculated using the following formula: Cell resistance rate (%) = (experimental group OD450 value/control group OD450 value) × 100.
Tunel assay
The rate of apoptosis was analysed by TUNEL assay by using detection kit (Boehringer Mannheim, Germany).
Statistical analysis
One-way analysis of variance (ANOVA) and student T-test was performed to determine the significant difference between the treatment and control groups. A probability level of p<0.01 was considered as statistically significance.
Results
FACs analysis of side population cells by hoechst 33342
Live cells were selected against Propidium Iodide in order to exclude the dead cells in both control and drug treated groups. We have identified about 3.3% of distinct cell population (SP cells) towards the SP-violet region of the dot plot of the FACS profile, which expels the Hoechst 33342 dye efficiently (Figure 1A). However upon treatment with verapamil (an inhibitor for ABC transporters), the prevalence of SP cells were significantly reduced to 0.3% (Figure 1A). Hence, these data suggest that ovarian carcinoma contains significant percentage of SP cells, which can efficiently pumps out the DNA binding dye via ABC transporters.
Enhanced expression of dysadherin in ovarian carcinoma SP cells
Next, the FACs sorted SP cells were subjected to evaluate the expression profile of dysadherin by RT-PCR and western blot. We found that relative mRNA expression of dysadherin and its protein level is significantly elevated in SP cells when compared to non-SP cells (Figures 1B and 1C). Similarly, the expression pattern of ABC transporter gene ABCG2 and stemness genes such as CD133, Oct-4, EpCAM were highly elevated in SP cells when compared to non-SP cells (Figures 1B and 1C). These findings suggest that elevated level of dysadherin in SP cells might play a major role in drug resistance and maintenance of self-renewal of SP cells.
Elevated expression of dysadherin enhances multidrug and apoptosis resistance of SP cells
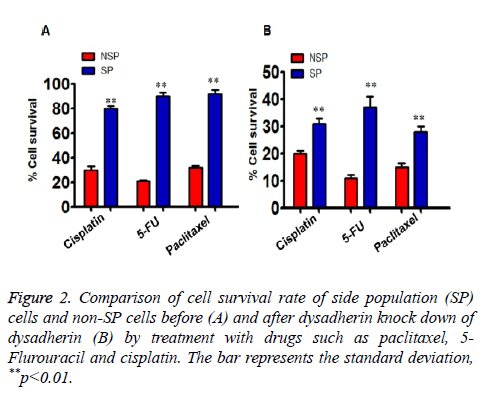
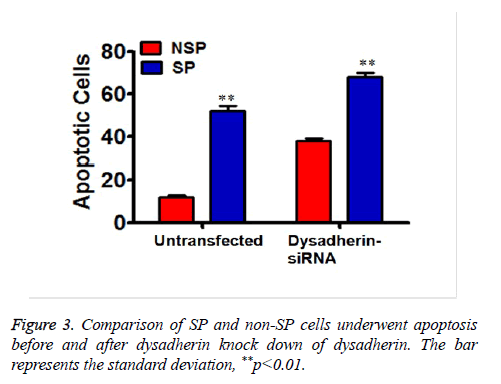
In order to address the significant role of dysadherin in SP cells, we used SiRNA technology to compromise dysadherin expression in SP cells. After silencing the dysadherin expression, the capacity of multi-drug resistance of SP cells was significantly reduced when compared prior to RNA interference SP cells. Before dysadherin knock down, the viability of SP cells are more than 80% even after treatment with chemotherapeutic drugs. However, the viability of SP cells was significantly reduced (less than 40%) in RNAi cells (Figure 2A, compare with 2B). Consequently, the number of SP cells subjected to apoptosis is significantly higher in dysadherin compromised SP cells (Figure 3).
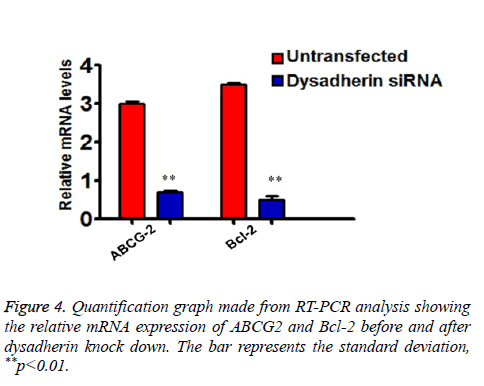
As we showed that RNA interference of dysadherin makes SP cells more sensitive to drug treatment and apoptosis, we subsequently checked the relative mRNA expression of ABC transporter and anti-apoptotic gene Bcl-2 in SP cells alone by RT-PCR. As shown in the graph (Figure 4), the transcriptional regulation of ABCG2 and Bcl-2 are significantly down regulated after silencing of dysadherin. These data suggest that enhanced dysadherin plays a crucial role in drug and cell death resistance of SP cells.
Discussion
A large number of studies reported that persistence of small subset of cancer stem cells (CSCs) or tumour-initiating cells (TICs) are responsible for treatment failure and tumour initiation and invasion, chemo resistance, and tumour recurrence [24,25]. Eradication of complete refractory of cancer stem cells are urgently needed to provide long term disease free survival. In such circumstances, understanding the molecular mechanism and downstream signalling pathways for drug and apoptosis resistance meditated by cancer stem cells is crucial one. Ovarian cancer is marked by a high degree of cellular heterogeneity and contains a cancer stem cell (CSC) population that contributes to tumour growth and treatment resistance [26-29]. Recent experimental evidences suggest that elevated levels of dysadherin had been observed in the stem cell enriched side populations in several cancers [17,18]. In a very recently published report, it has been reported that dysadherin is a potential driver for metastasis in ovarian carcinomas [19].
So far, Hoechst 33342-dye exclusion techniques are considered as a valuable technique for the purification of CSCs [30,31] SP cells has all the principal features of CSCs. Using Hoechst 33342-dye exclusion assay, 3.3% of side population cells were observed from ovarian carcinoma samples, whose prevalence was significantly reduced to 0.3% upon treatment with verapamil. Verapamil is an MDR1 transporter protein inhibitor and can efficiently block the drug efflux caused by the action of SP cells. This confirms that SP cells are present in ovarian carcinomas and have a role in the development of resistance to chemotherapeutic drugs via ABC transporters. Accumulating evidence from several solid tumours indicates that there is enhanced expression of different stem cell surface proteins in SP cells and that they are the major players in the maintenance of self-renewal [32,33]. In the present work it has also demonstrated that there is elevated expression of stem cell surface proteins such as CD133, Oct-4 and EpCAM in SP cells compared with non-SP cells.
Another remarkable feature of SP cells is the resistance to chemotherapeutic drugs. The conventional therapies are able to kill most of the neoplastic cells and not targeting the cancer stem cells. Therefore, the treatment escaped CSCs are become chemotherapy-resistant and high potent of initiating the tumour again. It was demonstrated that the survival rate of SP cells are significantly higher even after treatment with chemotherapeutic agents such as 5-FU, cisplatin and paclitaxel and that could be owing to the overexpression of ATP-binding cassette (ABC) drug transporters and Bcl-2, respectively. Dysadherin is a member of the FXYD family (FXYD5). It regulates NaKATPase, which is actively involved in maintaining cell permeability and polarity, which are essential for cell signalling and stem cell dynamics [34-36]. Further, it has been showed that SP cells from high-grade serous ovarian carcinoma displayed up-regulated transcriptional regulation of dysadherin in conjunction with elevated expression of ABCG2, stemness protein such as CD133, Oct-4 and EpCAM. After the expression of dysadherin is compromised, the SP cells are more sensitive to drug uptake and cell death, in addition to ABCG2 and the anti-apoptotic factor Bcl-2 being downregulated.
Conclusion
In summary, the present work suggests that unregulated transcriptional regulation of dysadherin might influence the expression of drug efflux pumps and anti-apoptosis pathways in SP cells. However, the precise molecular mechanism behind the chemo/apoptosis resistance of SP cells and their signalling pathways is still needs to be elucidated in provide more insights in to CSCs mediated tumour recurrence. Therefore, the available data suggest that development of novel anticancer drugs compromising dysadherin function could definitely help to improve the ovarian cancer treatment.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T. Cancer statistics. Cncr J clini 2008; 58: 71-96.
- Vecchione A, Belletti B, Lovat F, Volinia S, Chiappetta G, Giglio S. A microRNA signature defines chemoresistance in ovarian cancer through modulation of angiogenesis. Proc Natl Acad Sci U S A 2013; 110: 9845-9850.
- Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet 2014; 384: 1376-1388.
- Nam MS, Jung DB, Seo KH, Kim BI, Kim JH, Kim JH. Apoptotic Effect of Sanggenol L via Caspase Activation and Inhibition of NF-kappaB Signaling in Ovarian Cancer Cells. Phytother res 2016; 30: 90-96.
- Wang B, Liu SZ, Zheng RS, Zhang F, Chen WQ, Sun XB. Time trends of ovarian cancer incidence in China. Asian Pac J Cancer Prev 2014; 15: 191-193.
- Edwards SJ, Barton S, Thurgar E, Trevor N. Topotecan, pegylated liposomal doxorubicin hydrochloride, paclitaxel, trabectedin and gemcitabine for advanced recurrent or refractory ovarian cancer: a systematic review and economic evaluation. Health Technol Assess 2015; 19: 1-480.
- Ozols RF. Treatment goals in ovarian cancer. Int J Gynecol Cancer 2005; 15: 3-11.
- Kurman RJ, Shih I. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol 2011; 42: 918-931.
- Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 2010; 177: 1053-1064.
- Cannistra SA. Cancer of the ovary. N Eng J Med 2004; 351: 2519–2529.
- Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells 2006; 24: 3-12.
- Ramachandran C, Melnick SJ. Multidrug resistance in human tumors molecular diagnosis and clinical significance. Mol Diagn 1999; 4: 81-94.
- Ford JM, Hait WN. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev 1990; 42: 155-919.
- Sa´nchez-Garcı´a I, Vicente-Duen˜as C, Cobaleda C. The theoretical basis of cancer-stem-cell-based therapeutics of cancer: can it be put into practice? Bioessays 2007; 29: 1269-1280.
- Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA 2004; 101: 781-786.
- Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct side population of cells with high drug efflux capacity in human tumor cells, Proc Natl Acad USA 2004; 101: 14228-14233.
- Jiang N, Chen W, Zhang JW, Li Y, Zeng XC, Zhang T, Fu BS, Yi HM, Zhang Q. Aberrantly regulated dysadherin and B-cell lymphoma 2/B-cell lymphoma 2-associated X enhances tumorigenesis and DNA targeting drug resistance of liver cancer stem cells. Mol Med Rep 2015; 12: 7239-7246.
- Park JR, Kim RJ, Lee YK, Kim SR, Roh KJ, Oh SH, Kong G, Kang KS, Nam JS. Dysadherin can enhance tumorigenesis by conferring properties of stem-like cells to hepatocellular carcinoma cells. J Hepatol 2011; 54: 122-131.
- Raman P, Purwin T, Pestell R, Tozeren A. FXYD5 is a Marker for Poor Prognosis and a Potential Driver for Metastasis in Ovarian Carcinomas. Cancer Inform 2015; 14: 113-119.
- Ino Y, Gotoh M, Sakamoto M, Tsukagoshi K, Hirohashi S. Dysadherin, a cancer-associated cell membrane glycoprotein, down-regulates E-cadherin and promotes metastasis. Proc Natl Acad Sci U S A 2002; 99: 365-370.
- Nam JS, Hirohashi S, Wakefield LM. Dysadherin: a new player in cancer progression. Cancer Lett 2007; 255: 161-169.
- Fan J, Li R, Zhang R, Liu HL, Zhang N, Zhang FQ, Dou KF. Effect of Bcl-2 and Bax on survival of side population cells from hepatocellular carcinoma cells. World J Gastroenterol 2007; 13: 6053-6059.
- Shimamura T, Yasuda J, Ino Y, Gotoh M, Tsuchiya A, Nakajima A. Dysadherin expression facilitates cell motility and metastatic potential of human pancreatic cancer cells. Cancer Res 2004; 64: 6989-6995.
- Baccelli I, Trumpp A. The evolving concept of cancer and metastasis stem cells. J Cell Biol 2012; 198: 281-293.
- Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med 2011; 17: 313-319.
- Wiechert A, Saygin C, Thiagarajan PS, Rao VS, Hale JS, Gupta N, Hitomi M, Nagaraj AB, DiFeo A, Lathia JD, Reizes O. Cisplatin induces stemness in ovarian cancer. Oncotarget 2016.
- Abubaker K LA, Luwor R, Nazaretian S, Zhu H, Quinn MA, Thompson EW, Findlay JK, Ahmed N. Short-term single treatment of chemotherapy results in the enrichment of ovarian cancer stem cell-like cells leading to an increased tumor burden. Mol Cancer 2013; 12: 24.
- Burgos-Ojeda D WR, McLean K, Chen YC, Talpaz M, Yoon E, Cho KR, Buckanovich RJ. CD24+ Ovarian Cancer Cells Are Enriched for Cancer-Initiating Cells and Dependent on JAK2 Signaling for Growth and Metastasis. Mol Cancer Ther 2015; 14: 1717-1727.
- Ruan Z, Liu J, Kuang Y. Isolation and characterization of side population cells from the human ovarian cancer cell line SK-OV-3. Exp Ther Med 2015; 10: 2071-2078.
- Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res 2005; 65: 6207-6227.
- Robey RW, Shukla S, Finley EM, Oldham RK, Barnett D, Ambudkar SV, Fojo T, Bates SE. Inhibition of Pgp (ABCB1) and MDR associated protein-1 (ABCC1) mediate transport by the orally administered inhibitor CBT-1. Biochemical Pharmacol 2008; 75: 1032-1042.
- Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, Huang Z, Bentley RC, Mori S, Fujii S, Marks JR, Berchuck A, Murphy SK. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene 2009; 28: 209-218.
- Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, Ginestier C, Johnston C, Kueck A, Reynolds RK, Wicha MS, Buckanovich RJ. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res 2011; 71: 3991-4001.
- Garty H, Karlish SJ: Role of FXYD proteins in ion transport. Annu Rev Physiol 2006; 68: 431-459.
- Rajasekaran SA, Rajasekaran AK. Na, K-ATPase and epithelial tight junctions. Front Biosci 2209; 14: 2130-2148.
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol 2006; 22: 207–235.