ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 7
Development of the aesthetic orthodontic appliances using a silver plating process: the report on peel resistance
Nobukazu Shirakawa*, Toshio Iwata, Shinjiro Miyake, Takero Otsuka, So Koizumi, Takashi Usui and Toshitsugu Kawata
Orthodontic Division, Department of Oral Science, Kanagawa Dental University Graduate School, Yokosuka, Kanagawa, Japan
- *Corresponding Author:
- Nobukazu Shirakawa
Orthodontic Division, Department of Oral Science
Kanagawa Dental University Graduate School, Japan
Accepted date: December 26, 2016
The aim of this study was to analyse the peel resistance of silver plated wires examined in an intraoral setting. In this study, a 0.016 × 0.016 inch silver plated stainless steel wire and a 0.016 × 0.016 inch Teflon-coated nickel-titanium wire were investigated. The brackets of ceramic and stainless steel were bonded to the first and second bicuspids of two subjects. We investigated peel resistance of the wires three times, at the moment of setting, after 2 days, and after 1 week by using the following: 1) intraoral photographs, 2) stereoscopic microscope photographs, 3) color measurements, and 4) analysis of the surface properties of the silver plated wire after testing by scanning electron microscopy and Energy- Dispersive X-ray Spectroscopy (EDS). The silver plated wire turned black at the slot part of the brackets after 2 days but did not show peeling. The underlying metal was clearly exposed on the Teflon-coated wire. For the silver plated wire, the L* value decreased considerably 2 days after setting, while for the Teflon-coated wire, it decreased between 2 days to 1 week post setting. Sulphur atoms were detected on the silver plated wire. There was no significant peeling observed on the silver plated wire in between the bracket slots over a week. However, the wire turned black in the slot part of the brackets after 2 days.
Keywords
Silver plated, Peel resistance, Energy-dispersive X-ray spectroscopy.
Introduction
Appearance is one of patients’ main concerns during orthodontic treatment. Many orthodontic appliances have previously been composed of silver with a metallic color [1]. In response to increasing patient demands, researchers have developed transparent brackets made from ceramics or composites to improve the aesthetics of orthodontic appliances. They have also developed aesthetic archwires with polymer coating (Teflon or epoxy resin) [2,3].
Although these materials have excellent aesthetics, their functional properties are inferior to metal materials. Commonly used metal brackets are composed of stainless steel and have been shown to have the most ideal frictional characteristics [4]. Many researchers have shown increased frictional resistance, especially with polycrystalline ceramic and plastic brackets [5,6]. In addition, the color of coated archwires tends to change over time and the coating splits during oral use, exposing the underlying metal [7-9].
To address these deficiencies, we developed a surface plating treatment method that does not reduce the functional properties of metal nor exposes the underlying metal of archwires. Current plating methods mainly use alloys. Alloy plating can achieve various favourable colors depending on its composition, and it has wide-ranging applications as decorative plating. In addition, alloys are resistant to corrosion and wear, and are easy to melt during soldering [10].
Alloy plating is the precipitation of two or more types of metals and non-metals to make an alloy coating film during electroplating or other processes. We adopted an electroplating treatment. Electroplating methods can be applied to complexly shaped substrates, and can be used for mass production. In electroplating treatment, metal ions in the plating bath are deposited on the target metal surface as metal atoms and form a crystal lattice by surface diffusion.
In this study, we performed a silver alloy plating process on orthodontic archwires using an electroplating treatment method. The peel resistance of the silver plated wires set in the oral cavity was then analysed through intra-oral photographs, stereoscopic microscope photographs, and color measurement values. In addition, the surface properties of the silver plated wires after peel resistance testing were analysed using a Scanning Electron Microscope (SEM) and Energy Dispersive X-ray Spectrometer (EDS).
Materials and Methods
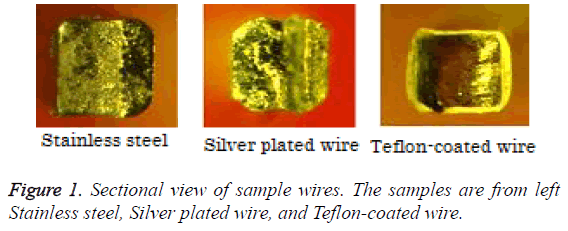
Silver plated wire
Typical alloy plating that can provide aesthetic properties include silver alloy and gold. Rhodium, platinum, and palladium alloys can also be utilized. Out of these, silver alloys more closely match natural teeth color; this can be achieved by adjusting the alloy composition. Silver plated wire was created through an electroplating process. The film thickness was set to 0.5 mm and shared almost the same dimensions of non-coated stainless steel wire. In contrast, the dimensions of the underlying metal of Teflon-coated wire decreased as the resin coating thickness increases (Figure 1). Therefore, silver plated wire was created based on a non-coated stainless steel wire that retained its dimensions.
Peel resistance
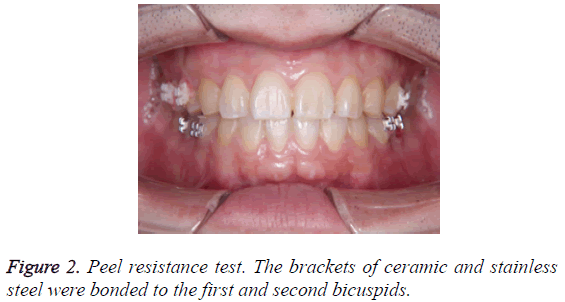
Two types of wires; 0.016 × 0.016 inch silver plated wire based on stainless steel and 0.016 × 0.016 inch nickel-titanium Teflon-coated wires were investigated in this study. The following procedure was performed on two subjects who consented to the study. The brackets of ceramic and stainless steel were bonded to the first and second bicuspids (Figure 2). Each wire was set to these brackets in the form of sections. We investigated the wires at three time points; the moment of setting, 2 days after setting, and 1 week after setting. Wire analysis comprised the following:
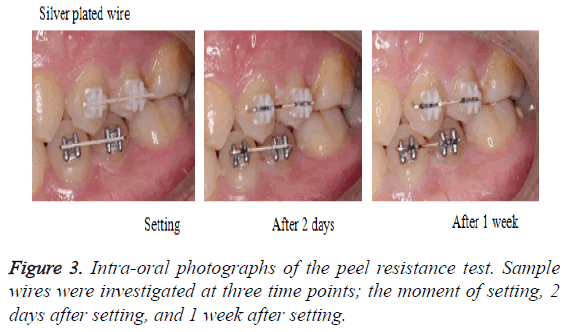
1. Intra-oral photographs: Photographs of the wires were taken when they were being set to the bracket (Figure 3).
2. Stereoscopic microscope photographs: The wires that were removed from brackets were cleaned with 80% alcohol and then air-dried to ensure an uncontaminated testing surface. Wire were then observed under a stereomicroscope (SZHILLB; Olympus, Tokyo, Japan) at 20 and 60X magnification (Figure 4).
3. Color measurements: We slightly modified the technique described by da Silva et al. [1]. Seven wire segments were placed tightly at both ends and fixed with wax (Figure 5). A colorimeter was then applied (Figures 6A and 6B) with the mean value of three readings of each sample recorded by the examiner. The color parameters were expressed using the Commission International de I’Eclairage L*a*b* color space systems. The L* value expresses the measure of lightness. The a* value expresses the measure of redness (+a*) or greenness (- a*). The b* value expresses the measure of yellowness (+b*) or blueness (-b*). The same color measurements were performed for each study time interval. We used the L* value from the measurement results for our evaluation. If the L* value is low, the color is dark, and if the L* value is high, the color is bright. The L* value is the easiest to understand numerical value in the evaluation of wire aesthetics.
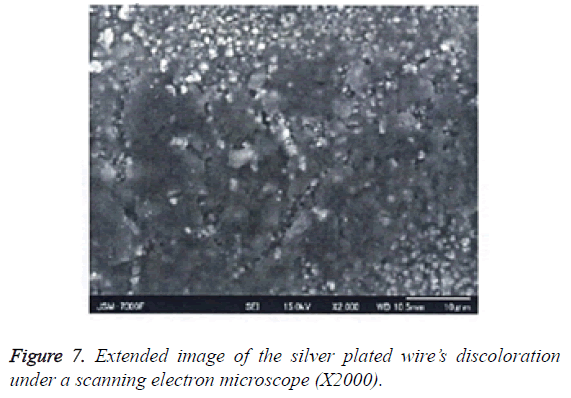
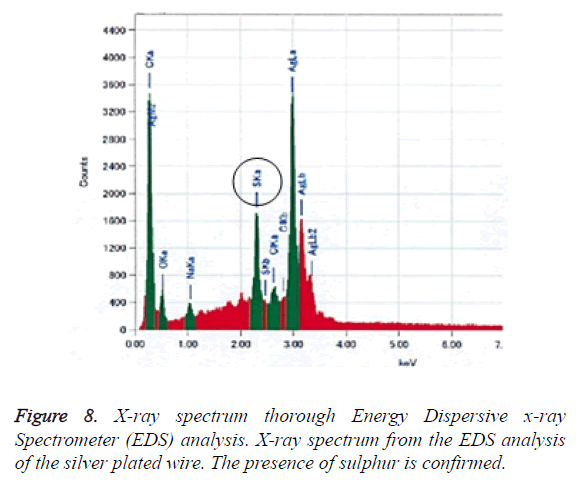
4. Surface properties of the silver plated wire after testing: After the peel resistance test, any changes to the silver plated surface were observed under SEM, and analysed by using EDX (Figures 7 and 8).
Results
Intra-oral photographs
Figure 3 shows the left side intra-oral view. The silver plated wire turned black only in the slot part of the bracket after 2 days. Its discoloration appeared more pronounced after 1 week. The Teflon-coated wire did not significantly change after 2 days.
However, after 1 week, although there were differences according to the part, significant peeling was observed between the bracket slots.
Stereoscopic microscope photographs
Figure 4 shows extended image photos of the wires’ surface. There was no peeling on the silver plated wire, just an area of discoloration. However, the underlying metal of the Tefloncoated wire was clearly exposed.
Color measurements
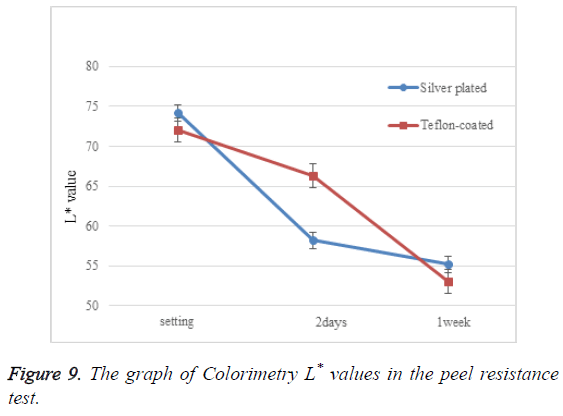
Table 1 and Figure 9 present numerical colorimetry values. L* value decreased considerably 2 days after setting, and between 2 days to 1 week after setting in the silver plated and Tefloncoated wires, respectively.
| Material | L* value | ||
|---|---|---|---|
| Wire | Setting | 2 days post-setting | 1 week post-setting |
| Silver plated | 74.2 ± 0.982 | 58.2 ± 0.992 | 55.2 ± 0.676 |
| Teflon-coated | 73 ± 0.839 | 66.3 ± 1.043 | 53 ± 0.962 |
Table 1: Colorimetry L* values in the peel resistance test.
Surface properties of the silver plated wire after testing
Table 2 shows the qualitative and quantitative results of the EDS analysis for the discolored area. Figure 7 shows the extended image of silver plated wire’s discoloration.
| C | O | Na | S | Cl | Ag | |
|---|---|---|---|---|---|---|
| Wt (%) | 26.2 | 9.4 | 1.1 | 6.5 | 1 | 55.8 |
| Atm (%) | 61.1 | 16.5 | 1.4 | 5.7 | 0.8 | 14.5 |
Table 2: Qualitative and quantitative energy dispersive x-ray spectrometer analysis of the discoloured area.
Figure 8 shows the X-ray spectrum from the EDS analysis relating to the silver plated wire’s discoloration. Sulphur atoms were observed on the surface of the wire; sulphur was not included in the original metal composition of the silver plated wire.
Discussion
Plated wire development
The use of aesthetic orthodontic appliances is increasing annually. Brackets have resulted in the use of ceramics or composites for appliance material, and wires are now coated with Teflon or epoxy resin. Changes in the functional properties due to such changes in material have various effects on orthodontic appliances, in particular frictional changes. Factors that affect frictional resistance include type of bracket, physical properties, size and alloy of arch wires, and method of ligation [11-17].
Many studies have compared the frictional resistance of different brackets; however, studies on the frictional influence of newly introduced ceramic brackets remain controversial [18-21]. To overcome this issue, it is necessary to apply archwire aesthetics without reducing the functional properties of the conventional metallic material. Therefore, we considered surface treatment methods for metals. These include plating treatment, spraying, coloring, and painting; we evaluated differences in bonding power among these methods. With the plating treatment, the base material is bonded to the plating film chemically, while in other methods the base material is bonded to the film physically. Chemical bonds are known to be much stronger than physical bonds. This is because chemical bonds are strengthened by the bonding force of electrons, whereas physical bonds are influenced by intermolecular forces. Therefore, in this study we decided to use a plating treatment method.
Peel resistance
Studies have shown that mechanical stimulus is the cause of archwire peeling [7-9]. The L* value of the Teflon-coated wire decreased considerably 2 days to 1 week after setting. This indicates that the underlying metal was directly exposed due to peeling in the Teflon-coated group. Such a reduction in aesthetics can occur frequently in reality, although it can vary according to the extent of patient’s malocclusion, quality of brushing, and lifestyle. There was no significant peeling between the bracket slots of the silver plated wire over 1 week. In silver plating treatment, the metal and the plating are chemically bonded together. In contrast, most of the Tefloncoating treatment occurs through an atomizing process with cleaned compressed air of the atomized Teflon particles [22]. Therefore, these differences (chemical bonding vs. mechanical coupling) between silver plating and Teflon coating may explain the variations in peeling.
Discoloration
A certain amount of sulphur was present in the area of discoloration. In general, chloridation by chlorine and sulfidation by sulphur result in silver discoloration, in particular silver is easily discolored when it comes into contact with a sulphur-containing gas. Discoloration observed in this study was limited to the bracket slots. Since brushing does not reach into the bracket slots, plaque can persist here. It is suggested that plaque remaining in bracket slots accelerate silver sulfidation over time. In the oral cavity, volatile sulphur compounds such as hydrogen sulphide, methyl mercaptan, and dimethyl sulphide can be present and can accelerate silver sulfidation. In the future, we intend to develop a silver plating treatment that strongly resists sulfidation.
Discoloration issues associated with silver plating highlighted in this study should be initially addressed. If an optimal plating technique is achieved, silver plating treatment can be applied to various dental treatment appliances. In future research, we will attempt to apply a silver plating treatment to not only wires, but also brackets, denture clasps etc. Based on the antibacterial performance of silver, this could also be applied to various medical appliances.
Conclusion
There was no significant peeling in between bracket slots of the silver plated wire 1 week after setting; however, the slot parts of the brackets were turned black 2 days after setting. No significant changes were observed in the Teflon-coated wire after 2 days, but significant peeling did occur between the bracket slots after 1 week.
Acknowledgements
The authors acknowledge Tomoyuki Yamamoto and Fumiko Oyama at Ebina Denka Kogyo Co., Ltd for support in preparing the silver plated wires and analysis of the wire’s surface properties.
References
- da Silva DL, Mattos CT, de Araujo MV, de Oliveira Ruellas AC. Color stability and fluorescence of different orthodontic archwires. Angle Orthod 2013; 83: 127-132.
- Yu B, Lee YK. Aesthetic colour performance of plastic and ceramic brackets-an in vitro study. J Orthod 2011; 38: 167-174.
- Lee YK. Colour and translucency of tooth-coloured orthodontic brackets. Eur J Orthod 2008; 30: 205-210.
- Kusy RP, Whitley JQ, Prewitt MJ. Comparison of the frictional coefficients for selected archwire-bracket slot combinations in the dry and wet states. Angle Orthod 1991; 61: 293-302.
- Bazakidou E, Nanda RS, Duncanson MG Jr, Sinha P. Evaluation of frictional resistance in aesthetic brackets. Am J Orthod Dentofacial Orthop 1997; 112: 138-144.
- Cha JY, Kim KS, Hwang CJ. Friction of conventional and silica-insert ceramic brackets in various bracket-wire combinations. Angle Orthod 2007; 77: 100-107.
- Elayyan F, Silikas N, Bearn D. Ex vivo surface and mechanical properties of coated orthodontic archwires. Eur J Orthod 2008; 30: 661-667.
- Postlethwaite KM. Advances in fixed appliance design and use: brackets and archwires. Dental Update 1992; 19: 276-280.
- Kusy RP. A review of contemporary archwires: their properties and characteristics. Angle Orthod 1997; 67: 197-207.
- Parker EA. Electroplating of gold alloys plating. Plating 1951; 38: 1134-1140, 1256-1259.
- Eslamian L, Borzabadi-Farahani A, Mousavi N, Ghasemi A. A comparative study of shear bond strength between metal and ceramic brackets and artificially aged composite restorations using different surface treatments. Eur J Orthod 2012; 34: 610-617.
- De Franco DJ, Spiller RE, Jr, von Fraunhofer JA. Frictional resistances using Teflon-coated ligatures with various bracket-archwire combinations. Angle Orthod 1995; 65: 63-72.
- Saunders CR, Kusy RP. Surface topography and frictional characteristics of ceramic brackets. Am J Orthod Dentofacial Orthop 1994; 106: 76-87.
- Guerrero AP, Guariza Filho O, Tanaka O, Camargo ES, Vieira S. Evaluation of frictional forces between ceramic brackets and archwires of different alloys compared with metal brackets. Braz Oral Res 2010; 24: 40-45.
- Williams CL, Khalaf K. Frictional resistance of three types of ceramic brackets. J Oral Maxillofac Res 2014; 4: e3.
- Omana HM, Moore RN, Bagby MD. Frictional properties of metal and ceramic brackets. J Clin Orthod 1992; 26: 425-432.
- Keith O, Jones SP, Davies EH. The influence of bracket material, ligation force and wear on frictional resistance of orthodontic brackets. Br J Orthod 1993; 20: 109-115.
- Read-Ward GE, Jones SP, Davies EH. A comparison of self-ligating and conventional orthodontic bracket systems. Br J Orthod 1997; 24: 309-317.
- Pizzoni L, Ravnholt G, Melsen B. Frictional forces related to self-ligating brackets. Eur J Orthod 1998; 20: 283-291.
- Schumacher HA, Bourauel C, Drescher D. The effect of the ligature on the friction between bracket and arch. Fortschr Kieferorthop 1990; 51: 106-116.
- Nucera R, Lo Giudice A, Matarese G, Artemisia A, Bramanti E, Crupi P. Analysis of the characteristics of slot design affecting resistance to sliding during active archwire configurations. Prog Orthod 2013; 14: 35.
- Farronato G, Maijer R, Carìa MP, Esposito L, Alberzoni D. The effect of Teflon coating on the resistance to sliding of orthodontic archwires. Eur J Orthod 2012; 34: 410-417.