ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 17
Detection of several serum factors in neonatal gonococcal conjunctivitis and its clinical significance
Huihui Zhu*, Bin Lu, Caiping Shi, Huaying Xie, Yanhong Ren and Jia Feng
Department of Ophthalmology, the Children’s Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, PR China
- *Corresponding Author:
- Huihui Zhu
Department of Ophthalmology
The Children’s Hospital
School of Medicine
Zhejiang University
Hangzhou, Zhejiang, PR China
Accepted on August 03, 2017
Objective: To investigate the changes of the levels of serum inflammatory factors, high sensitivity CReactive Protein (hs-CRP) and complement Component 3 (C3) and Component 4 (C4) in neonates with Neonatal Gonococcal Conjunctivitis (NGC), and to explore the clinical significance.
Methods: Thirty-two neonates with NGC including 14 boys and 18 girls (3 d to 6 d old) were selected in the Children’s Hospital of Zhejiang University to serve as experimental group, at the same time 32 healthy neonates (16 boys and 16 girls) with the same age distribution were also selected to serve as control group. There were no significant differences in age, gender and other basic information between two groups. Venous blood (2 ml) was extracted from all neonates with NGC and healthy neonates before and 3 d after the beginning of treatment, and on the day of discharge to prepare serum sample. Levels of hs-CRP, TNF-α, IL-1, IL-6, C3 and C4 were detected by Enzyme-Linked ImmunoSorbent Assay (ELISA).
Results: Before treatment, levels of serum hs-CRP, TNF-α, IL-1, IL-6, C3 and C4 in neonates with NGC were significantly higher than those in healthy neonates. With the progress of treatment, levels of hs- CRP, TNF-α, IL-1, IL-6, C3 and C4 were gradually reduced to reach normal level. Correlation analysis showed that levels of serum IL-6, C3 and C4 were positively correlated with the serum levels of hs-CRP.
Conclusion: Levels of serum hs-CRP, TNF-α, IL-1, IL-6, C3 and C4 were closely correlated with the development of NGC, and the detection of those factors can provide valuable information for the treatment of NGC.
Keywords
Neonatal gonococcal conjunctivitis (NGC), Inflammatory factors, High sensitivity C-reactive protein (hs- CRP), Complement.
Introduction
Although Gonococcal Conjunctivitis (GC) is rare in adults, the incidence is relatively high in neonates [1]. Compared with developed countries, GC is more common in less developed regions [2,3]. Neisseria gonorrhoeae are mainly transmitted in sexually active adults and adolescents through sexual act [4]. However, Neisseria gonorrhoeae can also infect neonates through their mothers during delivery [5]. Neonatal Gonococcal Conjunctivitis (NGC) usually occurs at 2 or 3 d after birth. The disease progress quickly, in some extreme cases, NGC can lead to corneal ulcers or even blindness. Antimicrobial therapy has been widely used in the treatment of GC for both neonates and adults [6]. However, the development of resistance of bacterial to antibiotic increases difficulties in the treatment of GC [7]. Due to the genetic diversity, Neisseria gonorrhoeae can obtain DNA from other gonococci, or even commensal Neisseria species at all stages of life cycle. This ability of Neisseria gonorrhoeae makes them more easily to develop resistance to antibiotics [8]. It has been reported that Neisseria gonorrhoeae can be resistant to most antibiotics that have been used in monotherapy, and Neisseria gonorrhoeae may become an untreatable disease in near future [8]. Even worse, a recent study pointed out that the spread of Neisseria gonorrhoeae with antibiotic-resistance became faster with more antibiotic treatment [9]. Therefore, how to control the use of antibiotics without affecting treatment efficacy has become a hot research field in the treatment of NGC.
Bacterial infection is always accompanied by the emergence of inflammatory response, and anti-inflammatory treatment can usually improve the conditions of disease [10,11]. Under the infection of Neisseria gonorrhoeae, expression levels of various pro-inflammatory factors will be increased, which in turn triggers inflammatory response to further aggregate the conditions [12,13]. In addition, the treatment progress of GC is always accompanied by the changes of inflammatory-related factors. Therefore, it will be reasonable to hypothesize that the monitoring of the changes of inflammatory-related factors will provide guidance for the use of antibiotics in treatment of NGC, so as to avoid drug abuse.
In this study, changes of inflammatory-related factors were monitored in neonates with NGC during the treatment with antibiotics. Clinical values of the detection of those factors for the treatment of NGC were also discussed. The report is as follows.
Materials and Methods
Objects
Thirty-two neonates with NGC including 14 boys and 18 girls (3 to 6 d old) were selected in the Children’s Hospital of Zhejiang University. The age of onset ranged from 24 h to 5 d and the duration of disease ranged from 3 to 6 d. At the same time, 32 healthy neonates (16 boys and 16 girls) with the same age distribution were selected as control group. No significant differences in age, gender and other basic information were found between two groups. Mothers of 20 neonates with NGC had a history of gonorrhea. Children showed varying degrees of eyelid swelling and conjunctival hyperemia, and a larger amount of pyogenic excretions with yellowish green color released from palpebral fissure can also be observed. Secretion of conjunctival sac was subjected to gram stain examination and bacterial culture. Patients with gram-negative bacilli identified in gram stain examination and/or Neisseria gonorrhoeae detected by bacterial culture were diagnosed as NGC. All 32 neonates were diagnosed with NGC. This study was proved by the Ethics Committee of our hospital. Parents of all the participants signed informed consent.
Treatment method
Conjunctival sac was rinsed with normal saline (about 50 mL/ time) and chloramphenicol eye drops (about 5 mL/time) in the morning, sterile cotton swab was used to remove eye discharge. All neonates with NGC were subjected to intravenous infusion of ceftriaxone sodium at a dose of 50 mg/kg/d, once per d, 7 d as a course of treatment. Local treatment was performed using ceftriaxone sodium eye drops (5 g/L) diluted in balanced salt solution. Local treatment was performed 3 times per d for 7 d as a course of treatment, and 4 to 5 eye drops were used for each time. Ofloxacin eye ointment was applied on eyes before bedtime. Discharge criteria: eyelid swelling faded, gram-negative bacilli and Neisseria gonorrhoeae were not detected. Negative results in 2 times of gram stain re-examination were considered as a cure.
Specimen collection
Venous blood (2 ml) was extracted from all neonates with NGC and healthy neonates before and 3 d after the beginning of treatment, and on the day of discharge. Blood samples were kept in room temperature for 2 h, followed by centrifugation (1000 rpm) for 20 min to collect serum. Serum was transferred to sterile EP tubes and stored at -80°C before use.
Enzyme-linked immunosorbent assay (ELISA)
Levels of Interleukin-1 (IL-1), Interleukin-6 (IL-6), Tumor Necrosis Factor α (TNF-α), high sensitivity C-Reactive Protein (hs-CRP), complement Component 3 (C3) and complement Component 4 (C4) were measured using ELISA kits provided by abcam (ab100562 for IL-1, ab46042 for IL-6, ab181421 for TNF-α, ab99995 for CRP, ab108822 for C3 and ab108825 for C4) and Bio-Rad Model 680 microplate reader (USA). Concentration of each protein was calculated using WLOGIT software.
Statistical analysis
Data were processed using SPSS19.0 software. Measurement data were expressed as ͞x ± s, comparisons within groups between different time points were performed by repeated measures analysis of variance, and comparisons between groups were performed by t-test. Correlation analyses were performed by Pearson correlation analysis. p<0.05 was considered to be statistically significant.
Results
Changes of hs-CRP levels in neonates with NGC during treatment
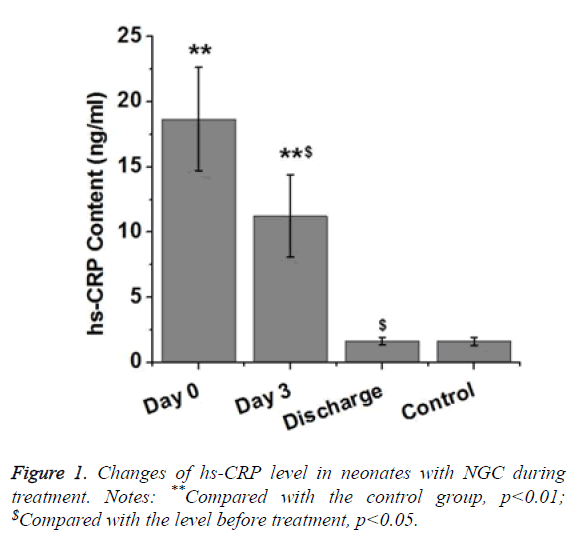
CRP is a protein synthesized by the liver. Level of CRP can be rapidly increased under inflammatory response. Therefore, CRP can be used as an indicator of bacterial infection. In this study, levels of hs-CRP in neonates with NGC were detected. As shown in Table 1 and Figure 1, level of serum hs-CRP in neonates with NGC was significantly higher than that in healthy neonates (p<0.01). Level of serum CRP in neonates with NGC was significantly reduced after 3 d treatment (p<0.05), but was still significantly higher than that of control group (p<0.01). After treatment, level of serum hs-CRP in neonates with NGC showed no significant difference to that of healthy neonates. Those results suggest that level of serum hs- CRP was gradually decreased to normal level (<2 mg/L) with the progress of treatment.
| Day | n | hs-CRP (ng/ml) |
|---|---|---|
| 0 | 32 | 18.67 ± 3.96** |
| 3 | 32 | 11.22 ± 3.15**$ |
| Discharge | 32 | 1.65 ± 0.27$ |
| Control | 32 | 1.63 ± 0.30 |
| Notes: **Compared with the control group, p<0.01; $Compared with the level before treatment, p<0.05. | ||
Table 1. Changes of hs-CRP levels in neonates with NGC.
Changes of TNF-α levels in neonates with NGC during treatment
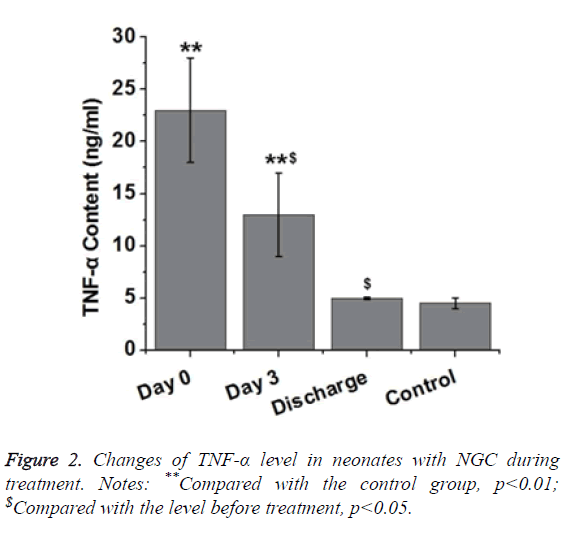
As an inflammatory factor, expression level of TNF-α can be increased by inflammatory response during the early stage of inflammation. Increased level of TNF-α protein can accelerate protein degradation to cause fever. TNF-α can also stimulate IL-1 and IL-6 secretion to trigger a series of cytokine cascades, thus causing secondary injury. Therefore, we investigated the changes of serum TNF-α level in neonates with NGC. As shown in Figure 2, level of serum TNF-α in neonates with NGC was significantly higher than that in healthy neonates (p<0.01). After treatment for 3 d, level of serum TNF-α in neonates with NGC was significantly reduced compared with the level before treatment (p<0.05), but was still significantly higher than that of control group (p<0.01). After treatment, level of serum TNF-α in neonate with NGC showed no significant difference to that of healthy neonates. Those results suggest that level of serum TNF-α was gradually decreased to normal level with the progress of treatment.
Changes of serum IL-1 and IL-6 levels in neonates with NGC during treatment
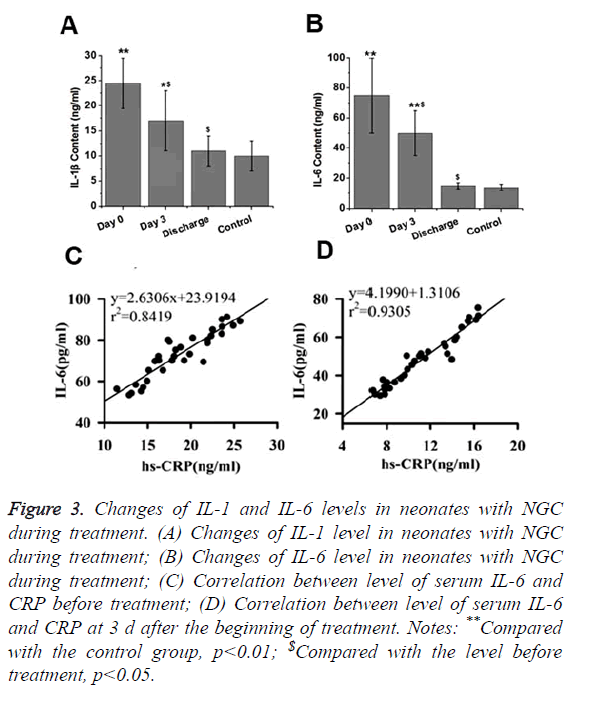
IL-1 and IL-6 are two pro-inflammatory factors that play pivotal roles in inflammatory responses. Therefore, changes of IL-1 and IL-6 levels in neonates with NGC during treatment were detected in this study. As shown in Figures 3A and 3B, levels of serum IL-1 and IL-6 in neonates with NGC were significantly higher than that in healthy neonates (p<0.01). After treatment for 3 d, levels of serum IL-1 and IL-6 in neonates with NGC were significantly reduced compared with the levels before treatment (p<0.05), but were still significantly higher than those of control group (p<0.05 or p<0.01). After treatment, no significant differences were found in levels of serum IL-1 and IL-6 between neonates with NGC and healthy neonates. Those results suggest that levels of serum IL-1 and IL-6 were gradually decreased to normal level with the progress of treatment.
Figure 3. Changes of IL-1 and IL-6 levels in neonates with NGC during treatment. (A) Changes of IL-1 level in neonates with NGC during treatment; (B) Changes of IL-6 level in neonates with NGC during treatment; (C) Correlation between level of serum IL-6 and CRP before treatment; (D) Correlation between level of serum IL-6 and CRP at 3 d after the beginning of treatment. Notes: **Compared with the control group, p<0.01; $Compared with the level before treatment, p<0.05.
IL-6 is an important factor in stimulating the production of CRP. Therefore, correlation between IL-6 level and CRP level was analyzed. Results showed that level of IL-6 was positively correlated with level of CRP at 3 d after the beginning of treatment (Figure 3C) and after the completion of treatment (Figure 3D).
Changes of levels of C3 and C4 in neonates with NGC during treatment
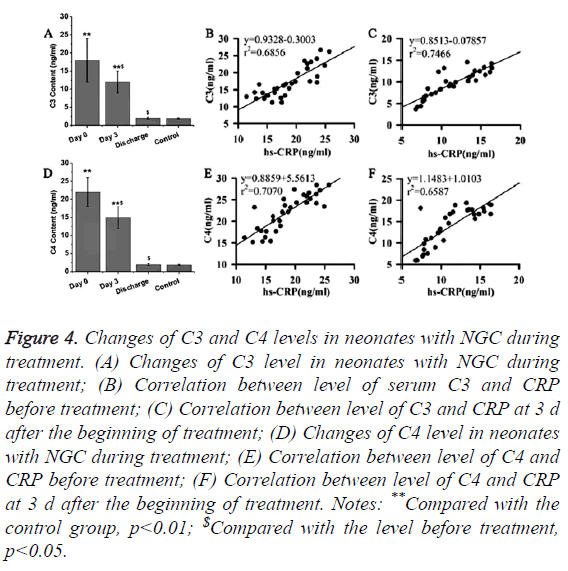
CRP can bind to complements to participate in immunoregulation. Therefore, changes of levels of C3 and C4 in neonates with NGC during treatment were detected in this study. As shown in Figures 4A and 4D, levels of serum C3 and C4 in neonates with NGC were significantly higher than that in healthy neonates (p<0.01). After treatment for 3 d, levels of serum C3 and C4 were significantly reduced compared with the levels before treatment (p<0.05), but were still significantly higher than those of control group (p<0.01). After the completion of treatment, no significant differences were found in levels of serum C3 and C4 between neonates with NGC and healthy neonates after the completion of treatment. Those results suggest that levels of serum C3 and C4 were gradually decreased to normal level with the progress of treatment. In addition, correlation analysis revealed that levels of serum C3 and C4 were positively correlated with the serum levels of hs- CRP (Figures 4B, 4C, 4E and 4F).
Figure 4. Changes of C3 and C4 levels in neonates with NGC during treatment. (A) Changes of C3 level in neonates with NGC during treatment; (B) Correlation between level of serum C3 and CRP before treatment; (C) Correlation between level of C3 and CRP at 3 d after the beginning of treatment; (D) Changes of C4 level in neonates with NGC during treatment; (E) Correlation between level of C4 and CRP before treatment; (F) Correlation between level of C4 and CRP at 3 d after the beginning of treatment. Notes: **Compared with the control group, p<0.01; $Compared with the level before treatment, p<0.05.
Discussion
With the ability to obtain DNA from other Neisseria species, Neisseria gonorrhoeae can easily develop resistance to antibiotics used in clinical practices. It has been reported that almost all commonly used antibiotics classes including, tetracycline, penicillin and fluoroquinolones failed in the treatment of Neisseria gonorrhoeae due to resistance [14,15]. Various vulnerable groups suffered from Neisseria gonorrhoeae [16,17]. In some countries, only one antibiotic can be used to effectively treat Neisseria gonorrhoeae [9]. Therefore, the development of a management program to reduce the use of antibiotic without affecting treatment efficacy is urgently needed. Inflammatory response, which is involved in the development of various bacterial infections, can contribute to the aggregation of disease conditions. Increases in level of CRP can usually be observed in patients with chronic or acute inflammation and bacterial infection [18]. Therefore, increased level of CRP protein is usually used as an indicator of bacterial infections in clinical practices. In the study of serious bacterial infection in neonates, Stein reported that the specificity of CRP was 82% for the prediction of infection, which was significantly higher than that of PCT (75%) and s- TREM-1 (48%) [19]. In another study, sensitivity and specificity of the use of CRP in predicting bacterial infection in liver cirrhosis patients reached 77% and 85%, respectively [20]. In the study of AIDS, CRP was proved to be a predictor of mortality in female patients with HIV-1 infection [21]. All those studies suggest the clinical values of CRP in predicting other types of infections. A recent study reported that expression level of CRP was also upregulated in patients with Gonococcal infection [22]. In our study, level of serum CRP was found to be significantly increased in neonates with NGC than in healthy neonates. In addition, level of serum CRP was gradually decreased to normal level with the progress of treatment. Those results suggest that CRP can potentially be used as an indicator of the efficacy of antibiotics in the treatment of NGC.
As an inducer of the inflammatory response, expression level of TNF-α is usually increased in the early stage of various infections [23]. During persistent fungal infection, increased release of TNF-α from inflammatory dendritic cells was proved to be able to increase IL-5/IL-17A levels in lung to promote inflammatory responses in neutrophilic airway [24]. It has been reported that expression level of IL6 was upregulated under fungal infection, and increased level of IL6 protein could regulate the interactions between IL-17A and IL-17RC to increase inflammatory responses induced by microbial infection [25]. The production of IL-1 was also enhanced during inflammation [26]. In contrast, levels of IL-1 and IL-6 were gradually reduced during the treatment of inflammation diseases [27,28]. Consistent results were found in our study. In this study, levels of TNF-α, IL-1 and IL-6 were found to be significantly higher in neonates with NGC than healthy neonates. With the progress of treatment, levels of serum TNF- α, IL-1 and IL-6 were gradually reduced to normal level. Those data suggest that TNF-α, IL-1 and IL-6 may potentially be used as predictors for the treatment outcomes of NGC. In addition, IL-6 was reported to be able to stimulate the expression of CRP. In addition, level of IL-6 was found to be positively correlated with the level of CRP, indicating the interactions between IL-6 and CRP during NGC.
CRP can usually bind to complement to play its role in inflammatory responses [29]. In this study, significantly increased levels of C3 and C4 (two components of complement) were observed in neonates NGC compared with healthy neonates. However, levels of C3 and C4 were gradually reduced to normal levels with the progress of treatment. Those results indicate that the detection of levels of C3 and C4 may also provide valuable information for the prediction of treatment outcomes of NGC. It’s worth to note that a single factor may not be sufficient for the accurate prediction of the efficacy of the treatment of NGC. A previous study showed that the sensitivity of CRP is only 45% in the prediction of serious bacterial infection in neonates. Thus, combined use of those factors may significantly increase the accuracy and efficiency.
In conclusion, increased levels of IL-1, IL-6, TNF-α, CRP, C3 and C4 were closely related with the development of NGC. The detection of those factors can provided valuable information for the treatment of NGC.
References
- McAnena L, Knowles SJ, Curry A. Prevalence of gonococcal conjunctivitis in adults and neonates. Eye 2015; 29: 875.
- Liang Y, Wang P. Influence of prudential value on the subjective well-being of Chinese urban-rural residents. Soc Indicat Res 2014; 118: 1-19.
- Liang Y, Guo M. Utilization of health services and health-related quality of life research of rural-to-urban migrants in China: A cross-sectional analysis. Soc Indicat Res 2015; 120: 277-295.
- Chow EPF, Lee D, Tabrizi SN. Detection of Neisseria gonorrhoeae in the pharynx and saliva: implications for gonorrhoea transmission. Sex Transm Infect 2016; 92: 347-349.
- Hammerschlag MR. Chlamydial and gonococcal infections in infants and children. Clin Infect Dis 2011; 53: 99-102.
- Xiridou M, Lugnér A, De Vries HJC. Cost-effectiveness of dual antimicrobial therapy for gonococcal infections among men who have sex with men in the Netherlands. Sex Transm Dis 2016; 43: 542-548.
- Lancaster JW, Mahoney MV, Mandal S. Update on treatment options for gonococcal infections. Pharm J Hum Pharmacol Drug Therap 2015; 35: 856-868.
- Dillon JAR, Parti RP, Thakur SD. Antibiotic resistance in Neisseria gonorrhoeae: will infections be untreatable in the future? Immunodeficiency 2016; 2: 5.
- Fingerhuth SM, Bonhoeffer S, Low N. Antibiotic-resistant Neisseria gonorrhoeae spread faster with more treatment, not more sexual partners. PLoS Pathog 2016; 12: e1005611.
- Shi J, Zhao Y, Wang Y. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014; 514: 187-192.
- Neely CJ, Kartchner LB, Mendoza AE. Flagellin treatment prevents increased susceptibility to systemic bacterial infection after injury by inhibiting anti-inflammatory IL-10+ IL-12-neutrophil polarization. PloS One 2014; 9: e85623.
- Calton CM, Wade LK, So M. Upregulation of ATF3 inhibits expression of the pro-inflammatory cytokine IL-6 during Neisseria gonorrhoeae infection. Cell Microbiol 2013; 15: 1837-1850.
- Château A, Seifert HS. Neisseria gonorrhoeae survives within and modulates apoptosis and inflammatory cytokine production of human macrophages. Cell Microbiol 2015.
- Ison CA, Town K, Obi C. Decreased susceptibility to cephalosporins among gonococci: data from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) in England and Wales, 2007-2011. Lancet Infect Dis 2013; 13: 762-768.
- Davies SC, Fowler T, Watson J. Annual report of the chief medical officer: infection and the rise of antimicrobial resistance. Lancet 2013; 381: 1606-1609.
- Liang Y, Yi Y, Sun Q. The impact of migration on fertility under China’s underlying restrictions: A comparative study between permanent and temporary migrants. Soc Indicat Res 2014; 116: 307-326.
- Liang Y. Satisfaction with economic and social rights and quality of life in a post-disaster zone in China: Evidence From earthquake-prone Sichuan. Dis Med Public Health Preparedness 2015; 9: 111.
- Pieri G, Agarwal B, Burroughs AK. C-reactive protein and bacterial infection in cirrhosis. Ann Gastroenterol 2014; 27: 113.
- Stein M, Schachter-Davidov A, Babai I. The accuracy of C-reactive protein, procalcitonin, and s-TREM-1 in the prediction of serious bacterial infection in neonates. Clin Pediatr 2015; 54: 439-444.
- Lin KH, Wang FL, Wu MS. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection in patients with liver cirrhosis: a systematic review and meta-analysis. Diagn Microbiol Infect Dis 2014; 80: 72-78.
- Feldman JG, Goldwasser P, Holman S. C-reactive protein is an independent predictor of mortality in women with HIV-1 infection. J Acquir Immune Defic Syndr 2003; 32: 210-214.
- Romiopoulos I, Pyrpasopoulou A, Varouktsi A. A rare case of disseminated pyogenic gonococcal infection in an immunocompetent woman. Case Rep Infect Dis 2016; 2016.
- Silva LCR, Ortigosa LCM, Benard G. Anti-TNF-α agents in the treatment of immune-mediated inflammatory diseases: mechanisms of action and pitfalls. 2010.
- Fei M, Bhatia S, Oriss TB. TNF-α from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proce Natl Acad Sci 2011; 108: 5360-5365.
- Taylor PR, Roy S, Leal Jr SM. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, ROR (gamma) t and dectin-2. Nature Immunol 2014; 15: 143-151.
- Tannahill GM, Curtis AM, Adamik J. Succinate is an inflammatory signal that induces IL-1 (bgr) through HIF-1 (agr). Nature 2013; 496: 238-242.
- Reis C, Da Costa AV, Guimarães JT. Clinical improvement following therapy for periodontitis: Association with a decrease in IL-1 and IL-6. Exp Therap Med 2014; 8: 323-327.
- Wang TM, Hsieh SC, Chen JW. Docosahexaenoic acid and eicosapentaenoic acid reduce C-reactive protein expression and STAT3 activation in IL-6-treated HepG2 cells. Mol Cell Biochem 2013; 377: 97-106.
- Molins B, Fuentes-Prior P, Adán A. Complement factor H binding of monomeric C-reactive protein downregulates proinflammatory activity and is impaired with at risk polymorphic CFH variants. Sci Rep 2016; 6.