ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Artificial Intelligent Techniques for Bio Medical Signal Processing: Edition-I
Coumarin of Angelicae dahuricae attenuates paraquat-induced lung injury in mice
1Department of Orthopedics, The No. 89 Hospital of Chinese People’s Liberation Army, Weifang, China
2Department of Pharmacy, Qingzhou people’s Hospital, Weifang,China
3Department of Medicine, Affliated Hospital of Weifang Medical University, Weifang, China
4Department of Gynecology, Affliated Hospital of Weifang Medical University, Weifang, China
- *Corresponding Author:
- Hui Wang
Department of Gynecology
Affliated Hospital of Weifang Medical University
Weifang, China
Accepted on March 9, 2017
Purpose: Paraquat is one kind of widely used herbicide with highly toxic compound, which is severely harmful for human and animals. Acute poisoning cases occurred frequently, and the fatality rate increases year by year because there is no effective antidote. This study investigated whether coumarin of Angelicae dahuricae can reduce paraquat induced lung damage in mice.
Material and Methods: We hypothesize that Coumarin of Angelicae dahuricae (CAD) may have a protective effect of Acute Lung Injury (ALI) in mice, so we adopt ALI-caused by paraquat injection infected mice models, observed the mice after PQ infected with CAD protection affects animal mortality rates and the pathological changes of lung tissue, as well as for various oxidase in lung tissue homogenate and influence of the content of inflammatory cytokines in the CAD on the ALI-induced by paraquat protection research. Adult male mice were chosen and divided into 3 groups-control, paraquat and paraquat+coumarin of Angelicae dahuricae, mice in paraquat and paraquat+coumarin of Angelicae dahuricae (120 mg/kg) group received paraquat (50 mg/kg) daily for three weeks.
Results: As expected, paraquat could lead to significant changes of shape and structure of the lung. Coumarin of Angelicae dahuricae attenuated paraquat induced morphological damage of lung of experimental animals.
Conclusion: Our results showed that coumarin of Angelicae dahuricae could decrease the toxic effect of paraquat in lung.
Keywords
Coumarin of Angelicae dahuricae, Paraquat, Lung injury, Inflammation, Oxidative stress path.
Introduction
Paraquat (referred to as 1, 1’-dimethyl-4, 4’- dipyridiumdichloride, PQ) is a common widely used, rapid herbicide in developing countries which has caused thousands of death accidentally or intentionally characterized by multi organ failure [1,2]. For people, oral 20-40 mg/kg PQ aqueous solution could be fatal, if not treated; mortality could be as high as 50%. According to the survey, lung become a critical target organ during the paraquat poisoning, and is the main cause of death compared with other organ dysfunctions [2,3]. The physiological hallmark about the ALI caused by paraquat is edema, interstitial inflammation, hemorrhage and proliferation of bronchial epithelial cell [4]. The mechanism of PQ poisoning is not yet fully understood by scientists and researchers, but now it is widely accepted that main potential mechanism of paraquat leading to toxicity is oxidative stress which directly damages the cells by oxygen free radicals or indirectly damage vital cellular constituents by inflammatory cells and fibroblasts resulting in lung failure [5,6]. Physiologically, acute lung injury caused by PQ poisoning show severe acute inflammatory response and neutrophilic alveolitis [7,8] and result in persistent respiratory failure, thereby increasing probability of multiorgan dysfunction and mortality [9].
Currently, the treatment of ALI induced by paraquat has no specific antidote. Support treatment is still the main method, and curative effect is poor. Some new methods, such as novel antioxidant, paraquat specific antibody, pulmonary surfactant, immunosuppressant, hemoperfusion and continuous venous hemofiltration have made some progress [10-14]. In recent years, many researchers have been trying to use traditional western medicine, Chinese medicine and combined therapy of Chinese and western medicine to treat ALI-induced by paraquat [15]. Park et al. reported the effect of sivelestat on acute lung injury in paraquat-intoxicated rats and they found that this drug can at least partially reduce lung injury by inhibiting neutrophil infiltration and TNF-α secretion [16]. Zhang and Ding’s research group thought and designed a study to investigate the protective effects of salidroside on rat ALIinduced by PQ via suppressing the expression of TGF-β1 [17] because the traditional Chinese medicines have the advantages of widely distributed, effective ingredients not easy to be lost, fewer side effects, their application in treatment of ALIinduced by PQ is increasingly favored by people in recent years.
Coumarins are an elite class of compounds which has varieties of therapeutic activities including antioxidant, antiinflammatory, antitumor, antiviral, antituberculosis and antimicrobial. Radix Angelicae dahuricae, is the roots of Angelicae dahuricae which is an important member of traditional Chinese Herbs (TCH). It is a well-known in China for treatment of colds, headaches, toothache, coryza, trauma and leukorrhea, etc. Recently, it is reported that the main exact coumarins of the Radix Angelicae dahuricae is to be a major of the herb which exerts a definite anti-inflammatory effect [18].
In this work, we hypothesize that Coumarin of Angelicae Dahuricae (CAD) may have a protective effect of ALI in mice, so we adopt ALI induced by paraquat injection infected mice models, observed the mice after PQ infected with CAD protection affects animal mortality rates and the pathological changes of lung tissue, as well as for various oxidase in lung tissue homogenate and influence of the content of inflammatory cytokines in the CAD on the ALI induced by paraquat protection research.
Materials and Methods
Experimental animals
Adult mice (male, body weight 18-20 g) were obtained from the Animal Centre of China Academy of Medical Sciences in Beijing (China) and they were kept in the controlled light room with a temperature of 20-24°C, whose cycle time of light/dark is 12 h. All mice were fed on a standard diet in the laboratory. The research protocol followed the rule of Animal Care and Use Committee at Xi’an Jiaotong University.
Chemicals
Paraquat was purchased from Sigma-Aldrich Trading Co., Ltd (Shanghai, China). Coumarins of Angelicae dahuricae (CAD) was extracted from the root of Radix Angelicae Dahuricae according to the reference by 65% ethanol. All other drugs and reagents were obtained from the above Sigma-Aldrich Trading Co., Ltd (Shanghai, China) unless otherwise stated. The source of chemicals and solvents used were analytically pure.
Dose regimen
Three groups were randomly set up for all mice: control, PQ and drug treatment group. These mice in control group received intraperitoneally injections with physiological saline. PQ group and drug treatment group were received injected intraperitoneally injection of 50 mg/kg PQ. CAD was used to rescue mice of PQ poisioning in drug treatment group. The drug treatment group mice were injected intraperitoneally 50 mg/kg of PQ followed by 120 mg/kg of CAD daily for 21 days.
Histopathology
One third of lung of mice were collected for histopathologic examination in all groups and the rest of the lung was kept under -80°C. The samples were then preserved in 10% neutralbuffered formalin and were embedded in paraffin, finally sectioned. Hematoxylin and Eosin staining (HE) was performed to determine the inflammatory changes of the lung tissue. Finally, the sections were examined with a light microscope.
Oxidation index determination
The rest of lung was homogenated and centrifugated for detection of other parameters according to the instructions of corresponding ELISA kits and Automatic microplate reader and instructions on the use of the ultraviolet spectrophotometer, respectively to the lung tissue homogenate of Malondialdehyde (MDA), lactate Dehydrogenase (LDH), Myeloperoxidase (MPO), interleukins 1β, 6, 8 and the determination of the content of TNF alpha indicators.
Western blotting
Homogenised the lung tissues of the three groups in lysis buffer containing protease inhibitors respectively, and used the Bradford reagent to determine the protein concentrations. Samples containing 30 μg protein per lane were electrophoresed by 10% SDS-Polyacrylamide Gels (PAGE), then transferred onto Polyvinylidene Fluoride (PVDF) membranes by electroblotting and incubated overnight at 4ºC. Next, the protein levels were detected by the use of dilutions of the primary antibodies. These membranes were incubated with the followed Horseradish Peroxidase (HRP)-conjugated secondary antibodies, meanwhile through an enhanced chemiluminescence reagent (Millipore, USA) the bound antibodies were visualized, and then were quantified via densitometry by using ChemiDoc XRS+ image analyzer (Bio- Rad, USA).
Statistical analysis
For all the qualitative data, their values were expressed as means ± Standard Deviation (SD). Comparisons between groups were analysed by using One-Way ANOVA analysis followed by LSD test. value of less than 0.05 was considered to indicate a statistically significant difference.
Results
External symptoms
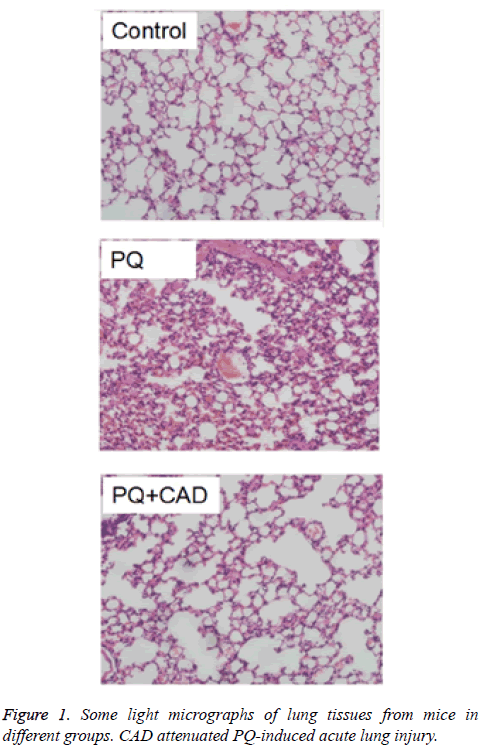
As shown in Figure 1A, control group of mice lung biopsy under light microscopy showed no apparent structural abnormalities. In the PQ model group (Figure 1B), when mice were treated with PQ, congestion and irregular structure arrangement were observed in the capillaries of alveolar walls, and the capillaries in the mice lung tissue also showed congested due to the significant increase of neutrophils.
Moreover, lung septa also became thickened. In addition, a large number of red blood cells appeared in alveolar cavities. While in the drug treatment group (Figure 1C), the pathological changes of the CAD intervention group and model group were similar, but there was a distinct improvement the inflammatory cell infiltration in the tissue stroma than the model group.
Effect of CAD treatment on MDA and MPO
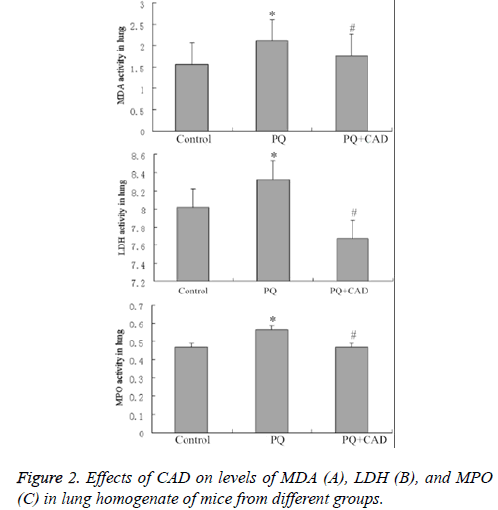
Compared with model group, in CAD intervention group the MDA vitality in lung tissue homogenate of mice was decreased significantly, but still higher than that in control group. In addition, MPO activity of the mice lung tissue in CAD intervention group could be significantly decreased, similar to the MDA vitality and also higher than that in control group (Figures 2B and 2C).
CAD against PQ-induced inflammation
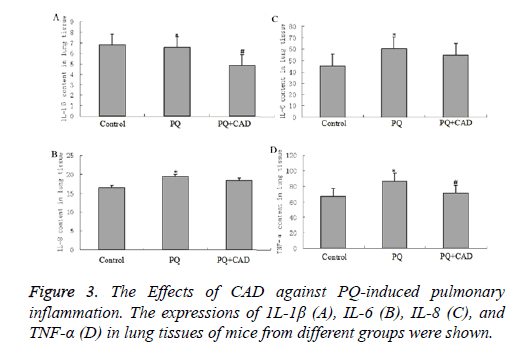
To detect effect of PQ on the inflammation factors, we examined the levels of several interleukins (that is IL-1β, IL-6 and IL-8) in the peripheral blood. Our data showed that the above inflammatory factors were obviously elevated in the PQ group compared with in the control group (seen as Figure 3,P<0.05). After PQ administration, the increased tendencies of IL-1β, IL-6 and IL-8 were significantly inhibited when the mice were treated with CAD (P<0.05), indicating that CAD could attenuate the inflammation reaction stimulated by PQ treatment.
CAD inhibited the expression of NF-κB and HIF-1α proteins in PQ-induced lung tissue
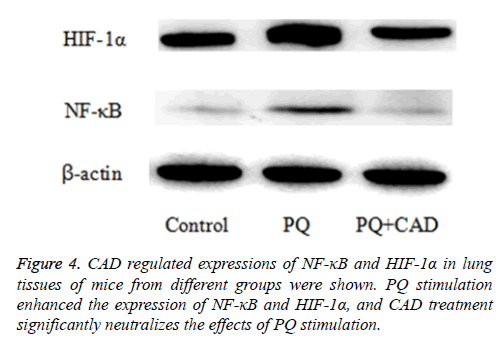
Next, in order to assess and explore the potential role of CAD in PQ-induced ALI, the authors also measured and determined the levels of NF-κB and HIF-1α proteins in the mice lung tissue of PQ-induced ALI mice by the mean of western blotting after paraquat challenge. The results displayed that the expressions of NF-κB and HIF-1α were increased by PQ stimulation, while notably; CAD pre-treatment could significantly reduce the expression of NF-κB and HIF-1α proteins in the injured lung tissues. The results are illustrated in the Figure 4.
Discussion
Paraquat (Gramoxone, 20% solution), whose lethal dose is 5-15 ml. It can be absorbed by the gastrointestinal tract, skin, and respiratory tract, resulting in lipid peroxidation of tissue membrane, especially lung tissue damage. The clinical manifestations were multiple system damage and high mortality rate. It is known that even tiny amounts of paraquat by oral ingestion can lead to irreversible lung and other organs’ damage and as yet refractory to any known therapeutic methods [19]. PQ-induced poisoning could lead to liver toxicity by testing the expression of liver enzymes, the level of jaundice and histopathological changes [20]. Some reports are about that pathological changes were also found in the liver and kidney at high doses of paraquat [21]. In addition, a mouthful of the paraquat used to induce pulmonary fibrosis and follows on death from renal tubular necrosis and circulatory failure [22]. Paraquat is accumulated in the epithelial cells of lung and kidney in several experiments, eventually leading to renal failure and pulmonary fibrosis [23]. In our study, the protective effect of CAD against toxic PQ was induced in some examined mice lungs. CAD prevents the subsequent histological damage of lung tissues. In a word, CAD maybe plays a chemopreventive role on paraquat poisoning. So, considering CAD with the capability of alleviating harmful effects of paraquat, it can be seen that CAD can improve the activity of antioxidant enzyme function of the body.
MDA, which as a product of lipid peroxidation can reflect degrees of reactive oxygen species attacking the cell in lung tissue indirectly. This, in turn, can show the antioxidant capacity of the body. Thus suggests that CAD can effectively reduce the content of MDA, prevent lipid peroxide, which can protect the body from oxidative damage. MPO is stored in neutrophils mainly, and is released outside of the cell in inflammation, reducing the activity of hydrogen peroxide, is an index reflecting the activity of neutrophils [24]. Our experiment shows that CAD could enhance the antioxidant capacity by decreasing the activity of MPO.
At present, the pathogenesis of lung injury caused by PQ poisoning possibly be the accumulation of this drug in the lung, the change of oxygen free radical, the mitochondrial damage caused by lipid peroxidation, imbalance of intracellular calcium content, inflammatory cells and immune cells, cytokines and chemokines, DNA damage in lung tissue [25]. Paraquat entered into the body and resulted in a series of stress response. Inflammatory mediators and other biological factors are activated [26]. These mediators play a big, important role in the pathogenesis of acute lung injury, pulmonary fibrosis and other pulmonary diseases induced by PQ [27]. Inflammatory markers in PQ infected experiment process, such as IL-1β, IL-6 as well as IL-8, can cause inflammatory response and result in white blood cell aggregation, the release of inflammatory medium, activation of inflammatory cells and immune cells. Our experiments have proved that paraquat could cause the secretion of inflammatory medium, resulting in the accumulation of immune cells and inflammatory cells infiltration. Our experiments also prove that CAD could enhance the antioxidant effect, reduce inflammatory factor and protect the body from oxidative damage.
According to reports, gene promoter regions of many fibrogenic factors also contain Nuclear factor (NF-κB) fixed nucleotide sequence [28] NF-κB as the hub link regulation of the cytokine network, can activate the gene transcription and expression of many inflammatory cytokines, and the formation of inflammation cascade so as to start lung inflammation. As one of the major transcription factors, NF-κB played an role in activating oxidative stress in the lung, which can up-regulate pro-inflammatory genes (T-helper 2 cytokines), and then lead to lung injury [29,30]. Our research found that paraquat could increase the activity of NF-κB and HIF-1α, so as to cause the body damage. Expectedly, CAD could down-regulate the expression of the above two, and thus improved paraquatinduced lung injury.
Conclusion
Our study demonstrated that CAD could reduce PQ-induced extra ALI in Mice. These results sufficiently indicated CAD’s inhibitory activities on the peroxide such as MDA, MPO, some pro-inflammatory mediators’ secretion as well as response of oxidative stress. Some further pharmacological and pathological evaluations are essential to elucidate the detailed mechanism between ALI via PQ-induced and the activity of CAD, which will provide a new potential drug and a novel approach in the treatment of this disease. Taken together, in our research experiment, the therapeutic effects of CAD on immune reaction and oxidative stress are preliminary discussed in PQ-caused acute lung injury mice model. Therefore, the future trend of research is to explore other routes of PQ on lung injury and the interaction and mutual connection among these routes. And also, the specific regulation mechanism remains to be further discussed. Furthermore, some studies should aim at refining the critical targets of CAD could lead to the pharmacological development of treatments for ALI induced by PQ.
References
- Liu S, Wang Q, Zhou R, Li C, Hu D. Hyperamylasemia as an early predictor of mortality in patients with acute paraquat poisoning. Med SciMonit 2016; 22: 1342-1348.
- Khodayar MJ, Kiani M, Hemmati AA, Rezaie A, Zerafatfard MR, RashidiNooshabadi MR. The preventive effect of atorvastatin on paraquat-induced pulmonary fibrosis in the rats. Adv Pharm Bull 2014; 4: 345-349.
- Chen T, Wang R, Jiang W, Wang H, Xu A. Protective effect of astragaloside iv against paraquat-induced lung injury in mice by suppressing Rho signaling. Inflammation 2016; 39: 483-492.
- Amirshahrokhi K. Anti-inflammatory effect of thalidomide in paraquat-induced pulmonary injury in mice. IntImmunopharmacol 2013; 17: 210-215.
- Awadalla EA. Efficacy of vitamin C against liver and kidney damage induced by paraquat toxicity. ExpToxicolPathol 2012; 64: 431-434.
- Mainwaring G, Lim FL, Antrobus K, Swain C, Clapp M. Identification of early molecular pathways affected by paraquat in rat lung. Toxicology 2006; 225: 157-172.
- Dinis-Oliveira RJ, Duarte JA, Sanchez-Navarro A, Remiao F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol 2008; 38: 13-71.
- Khalighi Z, Rahmani A, Cheraghi J. Perfluorocarbon attenuates inflammatory cytokines, oxidative stress and histopathologic changes in paraquat-induced acute lung injury in rats. Environ ToxicolPharmacol 2016; 42: 9-15.
- Mitsopoulos P, Suntres ZE. Cytotoxicity and gene array analysis of alveolar epithelial A549 cells exposed to paraquat. ChemBiol Interact 2010; 188: 427-436.
- Amirshahrokhi K, Khalili AR. Carvedilol attenuates paraquat-induced lung injury by inhibition of proinflammatory cytokines, chemokine MCP-1, NF-κB activation and oxidative stress mediators. Cytokine 2016; 88: 144-153.
- Chen BD, Lin QM, Lin SR, Chen F. Glucocorticoid combined with cyclophosphamide treating paraquat poisoning. China Medicine 2013; 8: 225-227.
- Javad-Mousavi SA, Hemmati AA, Mehrzadi S. Protective effect of Berberis vulgaris, fruit extract against Paraquat-induced pulmonary fibrosis in rats. Biomed Pharmacother 2016; 81: 329-336.
- Chen Y, Nie YC, Luo YL, Lin F, Zheng YF. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food ChemToxicol 2013; 58: 133-140.
- Podder B, Kim YS, Zerin T, Song HY. Antioxidant effect of silymarin on paraquat-induced human lung adenocarcinoma A549 cell line. Food ChemToxicol 2012; 50: 3206-3214.
- Zhang YC, Jiang YS, Wang ZQ. The protection effect of Rhubarb combine with Ulinastatin on paraquat poisoning induced lung injury in rats. Glob Trad Chinese Med 2016; 9: 795-797.
- Park JS, Park KH, Kim H, Choi SY. Effects of sivelestat treatment on acute lung injury in paraquat-intoxicated rats. Drug ChemToxicol 2014; 37: 114-120.
- Zhang Z, Ding L, Wu L, Xu L, Zheng L. Salidroside alleviates paraquat-induced rat acute lung injury by repressing TGF-β1 expression. Int J ClinExpPathol 2014; 7: 8841-8847.
- Bradberry SM, Watt BE, Proudfoot AT, Vale JA. Mechanisms of toxicity, clinical features, and management of acute chlorophenoxy herbicide poisoning: a review. J ToxicolClinToxicol 2000; 38: 111-122.
- Chen Y, Nie YC, Luo YL, Lin F, Zheng YF, Cheng GH. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food ChemToxicol 2013; 58: 133-140.
- Hong SY, Hwang KY, Lee EY, Eun SW, Cho SR, Han CS. Effect of vitamin c on plasma total antioxidant status in patients with paraquat intoxication. Toxicol Let 2002; 126: 51-59.
- Campbell S. Paraquat poisoning. ClinToxicol 1968; 1: 245-249.
- Yoshida A, Ohba M, Wu X, Sasano T, Nakamura M, Endo Y. Accumulation of platelets in the lung and liver and their degranulation following antigen-challenge in sensitized mice. Brit J Pharmacol 2002; 137: 146-152.
- Yang W, Thompson JW, Wang Z, Wang L, Sheng H, Foster MW. Analysis of oxygen/glucose-deprivation induced changes in SUMO3 conjugation using SILAC-based quantitative proteomics. J Proteome Res 2012; 11: 1108-1117.
- Reumaux D, de Boer M, Meijer AB, Duthilleul P, Roos D. Expression of myeloperoxidase (MPO) by neutrophils is necessary for their activation by anti-neutrophil cytoplasm autoantibodies (ANCA) against MPO. J Leukocyte Biol 2003; 73: 841-849.
- Liu F, Guan XD, Shang B. Research progress on mechanism and treatment of acute paraquat poisoning. J Toxicol 2011; 25: 380-383.
- Tong F, Tian YP, Shi HW. Study on the expression of nuclear factor-kappa B and inducible nitric oxide synthase in lung tissue of acute paraquat poisoned rats. Chinese J Crit Care Med 2006; 26: 520-522.
- Jian X, Ruan Y, Guo G, Zhang Y. Anti-TGF-beta1 antibody: an effective treatment for lung injury caused by paraquat in the future. Med Hypotheses 2008; 70: 705.
- Sun B, Chen YG. Advances in the mechanism of paraquat-induced pulmonary injury. Eur Rev Med PharmacolSci 2016; 20: 1597-1602.
- Tanabe T, Fujimoto K, Yasuo M, Tsushima K, Yoshida K. Modulation of mucus production by interleukin-13 receptor alpha2 in the human airway epithelium. ClinExp Allergy 2008; 38: 122-134.
- Yasuo M, Fujimoto K, Tanabe T, Yaegashi H, Tsushima K, Takasuna K. Relationship between calcium-activated chloride channel 1 and MUC5AC in goblet cell hyperplasia induced by interleukin-13 in human bronchial epithelial cells. Respiration 2006; 73: 347-359.