ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 11
Contrast-enhanced ultrasonography with a new contrast agent (SonoVue®) for characterization of renal tumors
Fei Wang1, Xinli Kang1, Song Cen1, Yuping Dai2, Chunhua Deng2 and Weifu Wang1*
1Department of Urology, People’s Hospital of Hainan Province, Haikou, PR China
2Department of Urology, the First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, PR China
- *Corresponding Author:
- Weifu Wang
Department of Urology
People’s Hospital of Hainan Province, PR China
Accepted date: April 10, 2017
Due to the increased use of imaging techniques such as ultrasound and computed tomography, an increasing number of renal tumors are being diagnosed incidentally. These tumors are often smaller and in an earlier stage, and it is difficult to distinguish benign from malignant tumors. The purpose of this study was to evaluate the value of Contrast-Enhanced Ultrasonography (CEUS) in the characterization of renal tumors. Fifty-six patients with renal tumors underwent CEUS examination using the contrast agent SonoVue® with the cadence Contrast Pulse Sequencing (CPS) technique. The duration of tumor and renal parenchyma enhancement after injection of the contrast agent were recorded, and the enhancement patterns during the cortical, parenchymal, and late phases were evaluated. The enhanced features of renal cell carcinoma and renal angiomyolipoma were displayed. Renal cell carcinoma showed hype- or iso-enhancement in the cortical phase and hypo-enhancement in the parenchymal and late phases. Renal angiomyolipoma showed hypo-enhancement during the entire contrast process. A thin, perilesional, rim-like hyper-enhancement in the late phase was the characteristic feature of renal cell carcinoma in CEUS. The diagnostic efficacy of CEUS in the characterization of renal tumors was as follows: sensitivity 91% (42/46), specificity 70% (7/10), positive predictive value 93% (42/45), negative predictive value 64% (7/11), and diagnostic accuracy 86% (49/56). Our results indicated that CEUS with SonoVue® might evaluate the vascularity of renal tumors in real time and provide useful information for their differential diagnosis and further treatment.
Keywords
Contrast-enhanced ultrasonography (CEUS), Renal tumors, Diagnoses
Introduction
The use of Ultrasonography (US) in mass screening has increased the number of incidentally discovered asymptomatic renal tumors [1,2]. Evaluation of the vascularity of renal tumors is important for their characterization and treatment. Conventional color and power Doppler sonography have a limited ability to depict intralesional vascularity because these methods are insensitive to slow flow and deeply located blood vessels [3]. Contrast-Enhanced Computed Tomography (CECT) is the accepted gold standard for the differential diagnosis of renal tumors. This modality is more sensitive than US in detecting a tumor thrombus in the renal vein and inferior vena cava or invasion of adjacent organs. Therefore, it is often used to determine tumor staging for further surgical treatment. However, disadvantages of CECT such as radiation exposure, risk of inducing severe renal dysfunction, and contraindication in patients allergic to iodine have severely limited its clinical application [4].
Contrast-Enhanced Ultrasonography (CEUS) is a new realtime technique in which the signals from circulating gas microbubbles can change parenchymal areas into enhanced, brighter tones in gray-scale imaging [5]. As it is more accessible and performable in all patients without regard for renal function state, it can overcome the limitations of conventional US and CECT and improve the assessment of vascularity in renal tumors. Unlike the contrast agents used in CECT and Magnetic Resonance Imaging (MRI), SonoVue® (Bracco Imaging Spa, Italy) is a second-generation ultrasound contrast agent consisting of phospholipid-stabilized microbubbles filled with sulfur hexafluoride. In contrast to SHU 508A (a first-generation contrast agent), SonoVue® allows real-time imaging due to the higher stability of the microbubbles, which contain an inert gas. As a blood pool agent, it has a more stable shell and more uniform diameter; additionally, its microbubbles do not diffuse through the vascular endothelium into the interstitium, and it can exhibit the vascularity of renal tumors more sensitively [6]. Although potentially a very useful imaging tool, CEUS with SonoVue® is still not widely used in urologic clinical practice. This study aims to evaluate the usefulness of CEUS with SonoVue® in the differential diagnosis of renal tumors using the Contrast Pulse Sequencing (CPS) technique.
Materials and Methods
Patient characteristics
Fifty-six consecutive patients (38 men and 18 women; 18-76 years of age; mean age, 42 years) with renal tumors first detected by conventional US underwent a CEUS examination prior to surgery. Patients were eligible if they were not younger than 18 years and not older than 80 years of age, and had no other serious medical conditions, such as cardiac insufficiency, coronary heart disease, or pulmonary hypertension, which would place them at high risk for complications associated with CEUS. Informed consent was obtained from all patients after the procedure and potential risk had been fully explained. The study was approved by the Human Studies Ethics Committee of the People’s Hospital of Hainan Province and the First Affiliated Hospital of Yat-Sen University in China.
Among the participants, 9 patients had gross hematuria, 16 had ipsilateral flank pain, and 31 with no symptoms had tumors incidentally detected by US examination. Only 2 patients were sensitive to percussion during physical examination. Every patient had a tumor, and a total of 56 renal tumors were evaluated. Among them, 24 tumors were located in the left kidney, 32 were located in the right kidney, and 13, 20, and 23 were located in the upper, middle, or lower pole of the kidney, respectively. All 56 tumors were histologically confirmed by radical (n=35) or partial (n=21) nephrectomy. The greatest transverse diameter of the tumors viewed ranged from 1.6 to 10.2 cm (mean ± SD, 4.7 ± 2.0 cm) on pathological examination. The pathologic diagnosis included renal cell carcinoma (n=44), metastasis from colon carcinoma (n=1), renal mesenchymal tissue malignant tumor (n=1), renal angiomyolipoma (n=7), renal multilocular cyst (n=1), renal adenoma (n=1), and renal xanthogranulomatous pseudotumor (n=1).
US contrast agent
The contrast agent used in this study was SonoVue®, a SF6- filled microbubble contrast agent stabilized by phospholipids. A dose of 2.4 ml of the contrast agent was injected into the antecubital vein as a bolus through a 20-gauge intravenous cannula (VenflonTM; Becton Dickinson, Franklin Lakes, NJ, USA), followed by a flush of 5 ml 0.9% sodium chloride solution.
Baseline and contrast-enhanced US examination
US examination was performed with a Sequoia 512 scanner (Siemens Medical Solutions, Mountain View, CA, USA) equipped with CPS, a contrast-specific CEUS software package. A 4 V1 vector transducer with a frequency range of 1-4 MHz was used. The kidney was thoroughly scanned using conventional gray-scale sonography to identify the target tumors. The location, size, shape, margin regularity, and internal echogenicity of each tumor were recorded. CPS was activated after contrast injection, and the range of the Morphology Index (MI) value shown on the screen was 0.15-0.20. The target tumors were observed continuously for at least 6 minutes after the contrast injection, and the process was recorded and stored on the hard drive in the scanner. All examinations were performed by two experienced sinologists who were unaware of the results of any imaging procedure except the sonographic findings.
Image analysis
Digital cine-clips of CEUS were retrospectively evaluated offline in consensus by two independent reviewers who were blinded to each patient’s identification, clinical history, histopathological results, and other imaging results. The duration of tumor and renal parenchyma enhancement after injection of the contrast agent were recorded. The patterns of contrast enhancement were classified as homogeneous enhancement (entire tumor enhanced uniformly), inhomogeneous enhancement (tumor enhanced at different levels), no enhancement, or peripheral nodular enhancement (peripheral enhancement with a nodular appearance). According to the experience acquired from CEUS of the liver and CECT of the kidney, the contrast procedure was divided into a cortical phase (7-30 s after contrast injection), parenchymal phase (31-60 s), and late phase (60-360 s). During various phases, the enhancement degree was classified as non-, hypo-, iso-, or hyper-enhanced in comparison with adjacent renal parenchyma.
Results
Enhancement features of renal tumors
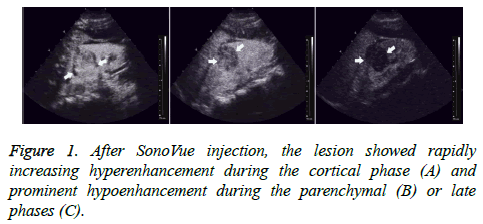
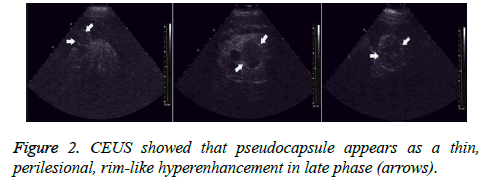
All 56 tumors showed various enhancements after contrast agent injection. During the cortical phase, Renal Cell Carcinomas (RCC) showed homogeneous enhancement in 27 (61.4%) tumors, inhomogeneous enhancement in 12 (27.3%) tumors, and no enhancement in the center, but peripheral nodular enhancement in 5 (11.3%) tumors. Thirty-five (79.5%) tumors showed hyper- or iso-enhancement, and 9 (20.5%) tumors showed hypo-enhancement. During the parenchymal and late phases, RCCs exhibited hypo-enhancement in 31 (70.5%) tumors; only 13 (29.5%) tumors showed hyperenhancement or iso-enhancement (Figure 1). A thin, perilesional, rim-like region of hyper-enhancement was seen in 36 (81.8%) tumors during the late phase (Figure 2). During the cortical phase, the enhancement of renal angiomyolipoma was inhomogeneous in 5 tumors and homogeneous in 2. It showed hypo-enhancement in 5 tumors and iso-enhancement in the remaining 2. During the parenchymal and late phases, 5 tumors faded quickly and showed hypo-enhancement; only 2 tumors washed out slowly and showed hyper-enhancement or isoenhancement. No thin, perilesional, rim-like hyperenhancement was seen in any tumor during the late phase.
Diagnostic efficacy of CEUS in characterization of renal tumors
There were 10 benign and 46 malignant tumors according to histopathologic diagnosis. Among them, 42 malignant and 7 benign tumors were correctly diagnosed by CEUS, and only 4 malignant and 3 benign tumors were misdiagnosed (Table 1). The diagnostic efficacy of CEUS in the characterization of renal tumors was as follows: sensitivity was 91% (42/46), specificity was 70% (7/10), positive predictive value was 93% (42/45), negative predictive value was 64% (7/11), and diagnostic accuracy was 86% (49/56).
| CEUS | |||
|---|---|---|---|
| Pathological diagnosis | n | Correct diagnosis | Misdiagnosis |
| RCC | 44 | 40 | 4 (RAML=2, Cystadenoma=2) |
| MRCC | 1 | 1 | 0 |
| RMTMT | 1 | 1 | 0 |
| RAML | 7 | 6 | 1 (RCC with tumor embolus) |
| RMC | 1 | 0 | 1 (Cystic RCC) |
| Renal adenoma | 1 | 0 | 1 (RAML) |
| RXP | 1 | 1 | 0 |
Table 1. Diagnostic efficacy of CEUS in the characterization of renal tumors.
Discussion
With the recent, rapid development of US contrast agents and great improvement in contrast techniques, CEUS has been widely used in the differential diagnosis of various kinds of diseases in solid organs such as the myocardium [7], liver [8], breast [9], and kidney [10], which are rich in blood supply. Numerous clinical trials have suggested that CEUS with SonoVue® can improve the sensitivity of US in the detection and characterization of liver lesions, and it may replace CECT and MRI to some extent in the differential diagnosis of focal liver lesions [8,11]. CEUS with SonoVue® may also become the modality of choice for the diagnosis of renal artery stenosis and detection of a perfusion deficit, as well as for the identification of infarctions and acute pyelonephritis, because US contrast agents can also improve the detection of abnormal macro- and microvascular disorders of the kidney [4,9].
The use of CEUS for imaging renal tumors was first described in 1994 in patients with RCC and renal insufficiency [12]. The researchers concluded that sonographic angiography, as they named it, is sufficiently sensitive for the detection of small tumors in patients with chronic renal failure. Later, Tranquart et al. [13] found that CEUS with SonoVue® can improve detection and characterization of renal tumors; the enhancement pattern of a tumor yielded important information for the differential diagnosis between benign and malignant renal tumors. However, Thorelius [14] and Correas et al. [15] reported that the enhancement pattern of renal cell carcinoma could not be visualized as clearly as with CECT, because its enhancement was very similar to that of the renal parenchyma. In 2007, Setola et al. [9] stated that CEUS should not be used for evaluating large solid renal masses because this technique does not usually supply additional relevant information to conventional US diagnosis and does not avoid further computed tomography and MRI evaluation and staging. However, small tumors may be better evaluated with addition of contrast enhancement, and the detection of diffuse tumor enhancement is a significant criterion in the differential diagnosis between benign and malignant hyperechoic tumors.
In view of this, we introduced the CPS imaging technique and utilized the new ultrasound contrast agent SonoVue® to observe the enhancement patterns of renal tumors in a continuous and dynamic manner. We found that RCCs showed rapidly increasing hyper-enhancement during the cortical phase and prominent hypo-enhancement during the parenchymal and late phases, which was in accordance with the results of Cai et al. [16] and Lu et al. [17]. More than 95% of RCCs exhibited later enhancement than adjacent renal tissue, and hyper- or isoenhancement was present in 79.5% tumors during the cortical phase. Homogeneous or inhomogeneous enhancement was the basic enhancement pattern during the cortical phase, and larger RCCs were prone to show no enhancement in the center and peripheral nodular enhancement due to tumor necrosis. This phenomenon agreed completely with Wink`s reports [18]. However, the enhancement of renal angiomyolipoma was slight and inhomogeneous in almost all tumors. Approximately 80% of tumors exhibited hypo-enhancement, and only 2 tumors showed iso-enhancement or hyper-enhancement during the contrast procedure.
There were 56 cases enrolled in our study, which consisted of 10 benign and 46 malignant tumors in histopathologic diagnosis. By using CEUS, 42 malignant and 7 benign cases were correctly diagnosed, and only 4 malignant and 3 benign cases were misdiagnosed. The diagnostic accuracy of CEUS in the differential diagnosis of renal tumors as benign or malignant improved to 86%. Therefore, our experience suggests that CEUS is a very useful tool for the detection and characterization of renal tumors, and it can effectively differentiate benign tumors from malignancies. Malignancy is suspected in cases of hyper-enhancement in the cortical phase and hypo-enhancement in the parenchymal and late phases and a benign tumor is highly suspected if the enhancement is lower than that of the adjacent renal tissue.
At the initial stage of our research, we had observed an unusual phenomenon: as the inner contrast agent increasingly faded out, a high-level echo loop began to appear around the tumor completely or incompletely during the contrast process. We classified it as one of 3 types according to its appearance: indistinctly seen (type I), incompletely seen (type II), or completely seen (type III). During the late phase, 36 high-level echo loops were observed in the RCC group while only 3 loops were observed in the non-RCC group. In addition, the highlevel echo loops in the former group were almost completely seen (type III 18, type II 9, type I 5), and the loops in the latter group were all indistinctly observed (type I 5). We postulate that the high-level echo loop might represent the tumor pseudocapsule, which is composed of fibrous tissue and compressed renal parenchyma. It is well known that the presence of a pseudocapsule is a useful sign for discriminating RCCs from other benign renal tumors, and it suggests the tumor is in an early stage and less aggressive [19]. Therefore, we performed parenchyma-sparing nephrectomy on 18 cases with completely high-level echo loop, which were confirmed as stage I by postoperative pathological results. Although several authors [20,21] have reported a low sensitivity of CEUS in detecting a pseudocapsule, our results have suggested that CEUS allowed identification of a pseudocapsule in RCCs with high accuracy, and the presence of a pseudocapsule is a major criterion for nephron-sparing surgery.
Conclusion
In conclusion, CEUS with CPS and SonoVue® permits detailed real-time characterization of the vascularity of renal tumors, which is useful both for differential diagnosis and for selection of conservative surgery for renal cell carcinoma.
Acknowledgements
This paper is supported by Natural Science Foundation of Hainan Province 2012 (No.812416) and Foundation of Hainan Provincial Health Department 2013.
Human Rights Statements and Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all patients for being included in the study.
Conflicts of Interest
All of the authors declare that they have no conflicts of interest regarding this paper.
References
- Filipas D, Spix C, Schulz-Lampel D, Michaelis J, Hohenfellner R. Screening for renal cell carcinoma using ultrasonography: a feasibility study. BJU Int 2003; 91: 595-599.
- Helck A, Danastasi M, Notohamiprodjo M, Thieme S, Reiser M, Clevert DA. Improved visualization of renal lesions using three-dimensional ultrasound-a feasibility study. Clin Hemorheol Microcirc 2011; 49: 537-550.
- Houtzager S, Wijkstra H, de la Rosette JJ, Laguna MP. Evaluation of renal masses with contrast-enhanced ultrasound. Curr Urol Rep 2013; 14: 116-123.
- Bertolotto M, Cicero C, Perrone R, Degrassi F, Cacciato F. Renal masses with equivocal enhancement at CT: characterization with contrast-enhanced ultrasound. AJR Am J Roentgenol 2015; 204: 557-565.
- Cosgrove D, Lassau N. Assessment of tumour angiogenesis using contrast-enhanced ultrasound. J Radiol 2009; 90: 156-164.
- ter Haar GR. Ultrasonic contrast agents: safety considerations reviewed. Eur J Radiol 2002; 41: 217-221.
- Li DY, Liang L, Xu TD, Zhang H, Pan DF, Chen JH, Chen J, Wang XP. The value of quantitative real-time myocardial contrast echocardiography for detection of angiographically significant coronary artery disease. Clin Cardiol 2013; 36: 468-474.
- Gerstenmaier JF, Gibson RN. Ultrasound in chronic liver disease. Insights Imaging 2014; 5: 441-455.
- Setola SV, Catalano O, Sandomenico F, Siani A. Contrast-enhanced sonography of the kidney. Abdom Imaging 2007; 32: 21-28.
- Wang XY, Kang LK, Lan CY. Contrast-enhanced ultrasonography in diagnosis of benign and malignant breast lesions. Eur J Gynaecol Oncol 2014; 35: 415-420.
- Lorusso A, Quaia E, Poillucci G, Stacul F, Grisi G, Cova MA. Activity-based cost analysis of contrast-enhanced ultrasonography (CEUS) related to the diagnostic impact in focal liver lesion characterisation. Insights Imaging 2015; 6: 499-508.
- Takase K, Takahashi S, Tazawa S, Terasawa Y, Sakamoto K. Renal cell carcinoma associated with chronic renal failure: evaluation with sonographic angiography. Radiology 1994; 192: 787-792.
- Tranquart F, Correas JM, Martegani A, Greppi B, Bokor D. Feasability of real time contrast enhanced ultrasound in renal disease. J Radiol 2004; 85: 31-36.
- Thorelius L. Contrast-enhanced ultrasound for extrahepatic lesions: preliminary experience. Eur J Radiol 2004; 51: 31-38.
- Correas JM, Claudon M, Tranquart F, Helenon AO. The kidney: imaging with microbubble contrast agents. Ultrasound Q 2006; 22: 53-66.
- Cai Y, Du L, Li F, Gu J, Bai M. Quantification of enhancement of renal parenchymal masses with contrast-enhanced ultrasound. Ultrasound Med Biol 2014; 40: 1387-1393.
- Lu Q, Huang BJ, Xue LY, Fan PL, Wang WP. Differentiation of renal tumor histotypes: usefulness of quantitative analysis of contrast-enhanced ultrasound. AJR Am J Roentgenol 2015; 205: 335-342.
- Wink MH, de la Rosette JJ, Laguna P, Lagerveld BW, Wijkstra H. Ultrasonography of renal masses using contrast pulse sequence imaging: a pilot study. J Endourol 2007; 21: 466-472.
- Wang L, Feng J, Alvarez H, Snarskis C, Gupta G. Critical histologic appraisal of the pseudocapsule of small renal tumors. Virchows Arch 2015; 467: 311-317.
- Ascenti G, Gaeta M, Magno C, Mazziotti S, Blandino A. Contrast-enhanced second-harmonic sonography in the detection of pseudocapsule in renal cell carcinoma. AJR Am J Roentgenol 2004; 182: 1525-1530.
- Magno C, Assenti G, Gali A, Caramia M, Anastasi G, Inferrena A, Melloni D. A new contrast agent for ultrasound imaging (Sonovue) to improve the detection of renal cell tumours pseudocapsule: Preliminary experience. Eur Urol 2003; 2: 221.