ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2012) Volume 23, Issue 4
Clinical efficacy of intravenous Cinepazide in the treatment of severe decompensated heart failure
Yingmin Lu1, DaminHuang1, Cunfang Dou1, Nengcai Yao1, Laixin Shi1, Xiaohan Luo1, Jinchun Zhang1, Jian Ji1, Junxian Shen1, Minmin Bao1, Ying Zhang2,Yigang Li1*
1Department of Cardiology, Xinhua(Chongming) Hospital of Shanghai Jiaotong University, Shanghai 202150, China
2 Department of Cardiology, Sixth People’s Hospital, Shanghai Jiaotong University, Shanghai 202150, China
- Corresponding Author:
- Yigang Li
Department of Cardiology Xinhua(Chongming) Hospital of Shanghai Jiaotong University, Shanghai 202150, China
Accepted date: June 12 2012
The present study aimed to investigate the effectiveness and safety of intravenous Cinepazide in the treatment of severe decompensated heart failure in patients who were unresponsive to traditional treatment with diuretics, angiotensin-converting enzyme inhibitors (ACEIs) and digitalis drugs. Patients with severe decompensated heart failure were recruited into this multicenter, randomized, controlled and parallel-group study and received treatment with either Cinepazide or Dobutamine therapy. In the Cinepazide group, a continuous infusion of Cinepazide was performed at 4 μg/kg·min for 23 h. In the control group, dobutamine was infused for 1 h at 2 μg/kg·min and then at 4 μg/kg·min for 23 h. The therapeutic effectiveness and safety were evaluated comprehensively. There were 120 patients in the Cinepazide group and 106 in the control group, all of whom received the evaluation of therapeutic effectiveness and safety. The effective rate was 31.9%(38/120) in the Cinepazide group and 17.9% (19/105) in the control group (P<0.01). After treatment for 24 h, the left ventricular ejection fraction (LVEF) was increased by 6.35% in the Cinepazide group and 4.6% in the control group (P>0.05). The mean stroke volume (SV) was increased by 11.1 ml in the Cinepazide group and 2.8 ml in the control group (P<0.05). The dyspnea and clinical manifestations were significantly improved in the Cinepazide group as compared to the control group. The plasma brain natriuretic peptide (BNP) level was significantly reduced after treatment with cinepazide (1997±865 pg/ml vs 384±114 pg/ml, P<0.005), and with dobutamine (1879±202 pg/ml vs 1025±48 pg/ml, P<0.005). Statistical analysis showed the significantly lower BNP level after treatment in the cinepazide group (P<0.005). No severe adverse effects were observed in either two groups. The incidence of adverse effects in the Cinepazide group was dramatically lower than in the control group (P <0.05). Adverse effects included hypokalemia, hypotension and premature ventricular contraction. When compared with Dobutamine, Cinepazide has definite effectiveness and higher safety in the treatment of severe decompensated heart failure, and all patients have favorable tolerance.
Keywords
Congestive heart failure; Cinepazide; brain natriuretic peptide; therapeutic outcome
Introduction
The incidence of heart failure is increasing with the population aging, and heart failure has been one of the most common diseases and one of the causes of death in the elderly. In China, the drugs for the treatment of heart failure include inotropic drugs, diuretics, vasodilators, angiotensin-converting enzyme inhibitors (ACEIs) and β- blockers. Cinepazide can inhibit the re-absorption of adenosine and the adenosine deaminase activity, delay the adenosine metabolism and then increase the endogenous adenosine content in local lesions resulting in the enhancement of the therapeutic effect of adenosine [1]. In addition, Cinepazide can inhibit the phosphodiesterase activity, decrease the platelet aggregation, reduce the production of free radicals, decrease the influence of neutroplils on the vascular endothelial cells and suppress the calcium overload, leading to the decrease of myocardial necrosis, protection of myocardial cells and improvement of cardiac hemodynamics [2,3]. In the present study, treatment with Dobutamine served as a control and the effectiveness and safety of intravenous Cinepazide in the treatment of acute or chronic heart failure of patients who were unresponsive to traditional treatments.
Patients and Methods
Patients
Patients with heart failure were recruited from November 2009 to November 2011 and randomly assigned into two groups at a ratio of 1:1: Cinepazide group and Dobutamine group. Inclusion criteria: 1) inpatients aged 18~75 years; 2) patients had grade III~IV left ventricular systolic dysfunction which was confirmed by the criteria for New York Heart Association (NYHA) classification; 3) the plasma BNP > 500 pg/ml; 4) Echocardiography confirmed LVEF<40%; 5) patients were unresponsive to traditional treatment with diuretics, ACEIs and digitalis drugs. Exclusion criteria: 1) patients with severe primary valvular heart diseases or pericardial diseases; 2) patients with uncontrolled thyroid diseases; 3) symptomatic patients with lung diseases who required long term treatment with theophylline or corticosteroids; 4) patients with arrhythmia; 5) supine systolic blood pressure <90 mm Hg (1 mm Hg=0.133 kPa) or >180 mmHg; 6) patients with liver and/or kidney dysfunction; 7) patients with cardiogenic shock or hypovolemia or unsuitable for vasodilator treatment; 8) pregnant or breast-feeding woman; 9) patients participating other studies within 3 months; 10) Endocrinopathies and infective states or other type of interacting drug usage have been excluded.
Drug administration
Cinepazide injection (12.5 mg/5 ml) was provided by the Beijing Fourth Ring Biopharmaceutical Co., Ltd, and dobutamine injection (20 mg/2 ml) by Shanghai No. 1 Biochemical & Pharmaceutical Co. Ltd. The Batch Number was recorded during the treatment.
In the screening phase, patients received physical examination and laboratory examination and the medical record was reviewed. In the treatment phase, eligible patients were randomly assigned into Cinepazide group and Dobutamine group. In the Cinepazide group, patients were intravenously infused with Cinepazide at 4 μg·kg- 1·min-1 for 23 h. Patients in the Dobutamine group received intravenous infusion of Dobutamine 2 μg·kg- 1·min-1 for 1 h and then at 4 μg·kg-1·min-1 for 23 h. The vital signs were observed including the symptoms of dyspnea, rales in the lungs, Jugular vein distention, hepatomegaly, lower extremity edema and fatigue. At the end of treatment, laboratory examination, echocardiography and echocardiography for left ventricular ejection fraction (LVEF) and stroke volume (SV). In some patients in the Cinepazide group, a Swan-Ganz catheter was inserted to measure the pulmonary capillary wedge pressure (PCWP) at 2, 4, 6 and 24 h after treatment.
Evaluation of clinical efficacy
The clinical effective rate ([patients with effectiveness/all patients] ×100%) and clinical benefit rate ([patients with effectiveness + patients with improvement/all patients] ×100%) were calculated. The clinical efficacy was determined as follows: significant improvement of symptoms and signs after treatment for 24 h and one of the following items were used to define the effectiveness: 1) LVEF was improved by ≥25% or returned to normal; 2) SV was increased by ≥25% or returned to normal; 3) PCWP was decreased by ≥25% or returned to normal; 4) cardiac index was improved by ≥25% or returned to normal. The improvement of above parameters <25% was defined as improvement. The SV and LVEF were compared between Cinepazide treated patients and dobutamine treated patients. According to the dyspnea and systemic symptoms, the outcome was graded as significant improvement, moderate improvement, mild improvement, no improvement, mild deterioration, moderate deterioration, and significant deterioration, which were scored from 1 to 7. The BNP level was compared between groups. In the Cinepazide group, the effect of Cinepazide treatment on the PCWP was examined.
Evaluation of safety
The number of patients withdrawing from the study, the incidence of adverse events, vital signs and findings in laboratory examination and electrocardiography were employed to evaluate the safety of different treatments. In the laboratory examination, routine blood test, urine test, and detections of liver function, kidney function, blood lipid, electrolytes and glucose were carried out. Echocardiography was performed to measure LVEF and SV. For patients of child-bearing age, urine pregnancy test was also done.
Statistical analysis
Statistical analysis was performed with SAS and data were expressed as mean ± standard deviation. Quantitative data were analyzed with t test and qualitative data with chi square test. Analysis of variance and CMH method with consideration of center effect were used to compare the efficacy between groups. The overall effective rate was analyzed with CMH method with consideration of center effect. A value of two-sided P<0.05 was considered statistically significant.
Results
Demographic information
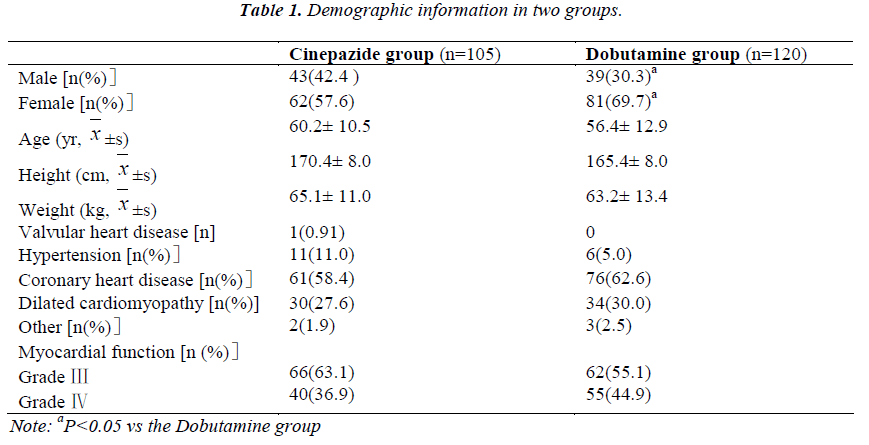
A total of 230 patients were recruited and 5 withdraw before study. Thus, 225 patients completed the present study and there were 105 patients in the Cinepazide group and 120 in the Dobutamine group. No significant differences were observed between groups in the age, height, body weight, and diseases but not the gender constitution (Table 1).
Clinical efficacy
Analysis of overall efficacy
In the Cinepazide group, the clinical effective rate was 31.9% (38/120), 54 had improvement and 28 were unresponsive to the treatment. In the Dobutamine group, the clinical effective rate was 17.9% (19/105), 46 had improvement and 40 were unresponsive to the treatment. Significant difference was found in the clinical effective rate between groups (P =0.005). The benefit rate was 77.3% (92/120) in the Cinepazide group and 61.3% (65/105) in the Dobutamine group showing signifiant difference (P =0.003).
Analysis of cardiac function
1) Improvement of LVEF: In the Cinepazide group, the mean LVEF was 30.5% and 36.9% before and after treatment, respectively. The LVEF was increased by 6.4% (95%CI: 4.74-7.96). In the Dobutamine group, the mean LVEF was 32.5% and 37.0% before and after treatment, respectively. The LVEF was increased by 4.6% (95%CI: 3.07~6.06). There was no significant difference in the improvement of LVEF between groups (P =0.117). When compared with LVEF before treatment, the relative increase (percentage) of LVEF was 24.0% (95%CI: - 27.78-118.15) in the Cinepazide group and 15.0% (95%CI: -19.44-74.3) in the Dobutamine group showing no significant difference (P >0.05). 2) Improvement of SC: In the Cinepazide group, the mean SV was 64.4 ml and 75.0 ml, and the increase of SV was 11.1 ml (95%CI: 6.62~15.61). In the Dobutamine group, the mean SV was 66.2 ml and 68.7 ml, and the increase of SV was 2.8 ml (95%CI: -1.38~7.03). Significant difference in the increase of SV was noted between two groups (P =0.010). The percentage increase of SV in the Cinepazide group and the Dobutamine group was 24.9% (95%CI: 15.70~34.15) and 9.2% (95%CI: 3.46~15.01), respectively, showing dramatic difference (P=0.008). 3) Improvement of dyspnea and systemic symptoms: the significant improvement of dyspnea was found in 23.5% of patients in the Cinepazide group and 10.9% in the Dobutamine group showing significant difference (P<0.05). The proportion of patients with significant improvement of systemic symptoms was 6.9% and 3.1% in the Cinepazide group and Dobutamine group, respecttively (P<0.05). In addition, the moderate improvement was found in 12.1% of patients in the Cinepazide group and 6.1% in the Dobutamine group (P<0.05). 4) In the Cinepazide group, the plasma BNP level was significantly decreased (1997±865 pg/ml vs 384±114 pg/ml, P<0.005). Pronounced decrease of BNP level was also noted after Dobutamine treatment (1879±202 pg/ml vs 1025±48 pg/ml, P<0.005). Moreover, the BNP level after treatment in the Cinepazide group was significantly reduced when compared with the Dobutamine group (384±114 pg/ml vs 1025±48 pg/ml, P<0.005). 5) Improvement of PCWP: PCWP was measured in a total of 13 patients in the Cinepazide group. Results showed the PCWP before treatment was 23.4±10.9 mmHg. 2, 4, 6 and 24 h after treatment, the PCWP declined to 17.9±9.7 mmHg, 18.0±9.7 mmHg, 17.7±9.0 mmHg and 17.3±9.0 mmHg, respectively. This findings suggest the PCWP decrease can maintain for at least 24 h, but the PCWP level after treatment was not statistically different from that before treatment (P=0.124).
Safety
The incidence of adverse effects was 13.5% (16/119) and 22.6% (24/106) in the Cinepazide group and the Dobutamine group, respectively showing significant difference (P<0.05). Study was interrupted due to adverse effects in 3 patients in the Cinepazide group: 1 with hypotension, 1 with ventricular fibrillation and hypokalemia and 1 with chest pain. In the Dobutamine group, 12 patients stopped the study due to adverse effects: 5 with palpitation, 4 with tachycardia, and 3 with gastrointestinal symptoms. The symptoms resolved after treatment discontinuation or Symptomatic treatment. In the Cinepazide group, the common adverse effects included hypokalemia, hypotension and premature ventricular contraction.
Discussion
Heart failure has been a common disease to date. In the developed countries, the incidence of heart failure is 1~2% in adults and 6~10% in subjects aged >65 years [4]. Framingham study showed the mortality of heart failure patients was 37% in males and 33% in females [5]. Currently, heart failure has become a public health problem threatening the human life due to high mortality and disability. Thus, it is imperative to improve the diagnosis and treatment of heart failure.
Acute decompensated heart failure can be classified into acute deterioration of chronic heart failure and acute onset of heart failure in the absence of history of heart failure. In early 2005, European Society of Cardiology released the first guideline for the diagnosis and treatment of acute heart failure [6], which provides guidance for the clinical diagnosis and treatment of heart failure. Currently, the treatment of acute decompensated heart failure is carried out with intravenous diuretics, vasodilators and inotropic drugs to maintain the stable hemodynamics and improve the symptoms. Once they are unresponsive, left ventricular assist device or surgical intervention is usually employed for the treatment [4,7].
β-adrenoceptor agonists and phosphodiesterase inhibitors are the common traditional inotropic drugs, but long term treatment with these drugs may increase the myocardial oxygen consumption, affect the myocardial relaxation, induce arrhythmia and raise the mortality [8]. Thus, it is necessary to develop novel drugs that not only improve the myocardial contraction but do not increase the myocardial oxygen consumption and the incidence of arrhythmia. As an adenosine synergist, Cinepazide meets the above requirements. Cinepazide has moderate calcium antagonistic effect and can dilate the coronary artery and decrease the pulmonary vascular resistance, which effectively reduces the MPAP and PCWP and increases CO. Cinepazide can also inhibit the phosphodiesterase activity, increase the cyclic adenosine monophosphate content in the myocardium and improve the myocardial contraction leading to the improvement of myocardial hemodynamics. There is evidence showing that Cinepazide can enhance the myocardial contraction and decrease the myocardial oxygen consumption [2] . Our results also showed Cinepazide could improve the myocardial function and reduce the myocardial oxygen consumption, which may be attributed to the decreased oxygen consumption and reduced adenosine triphosphate due to the decrease of ventricular wall tension and myocardial load, reduction of the response of heart to catecholamine and reduction of renin release [2]. Furthermore, Cinepazide may also inhibit the platelet aggregation, reduce the free radical production, decrease the adhesion of neutropils to the endothelial cells and inhibit the calcium overload [3], which reduces the myocardial necrosis, protects the myocardium and improves myocardial hemodynamics. Our results revealed the proportion of patients who met the criteria for endpoint was 28% in the Cinepazide group and 15% in the Dobutamine group. In the present study, the symptoms and myocardial function were used for the evaluation of the therapeutic efficacy in patients with severe acute heart failure. Our results showed the clinical effective rate in the Cinepazide group was 31.9% at 24 h after treatment, but that was only 17.9% in the Dobutamine group showing significant difference between groups.
Moreover, the safety of Cinepazide is superior to that of Dobutamine and other phosphodiesterase inhibitors. The incidence of headache was 2~9% and that if hypotension was 5%. Both headache and hypotension are the common side effects and frequently noted in the treatment with high dose. However, the incidence of arrhythmia following Cinepazide treatment was found to be similar to Dobutamine [1]. Other side effects included tachycardia, reduction of hematocrit and hemoglobin and hypokalemia, which might be secondary to the vasodilation and neuroendocrine activation. Similar findings were observed in the present study and confirmed the safety of Cinepazide in clinical practice.
BNP is a physiologically active substance that can exert diuretic, natriuretic and vasodilatic effects. It is mainly secreted by the ventricular myocytes. The BNP level can objectively reflect the degree of heart failure and is closely related to the cardiac function and LVEF [9]. The half-life of BNP is extremely short (about 20 min) and decreases rapidly following effective treatment [10-13]. Thus, the plasma BNP level is critical for the diagnosis of acute heart failure and evaluation of therapeutic efficacy. In the present study, our results showed the mean plasma BNP level increased significantly in patients with acute heart failure, which decreased significantly after Cinepazide treatment. The decrease of BNP level in the Cinepazide group was more obvious than in the Dobutamine group. Cinepazide can improve both the symptoms and hemodynamics. Cinepazide has the promise to replace the traditional inotropic drugs and become an important component in the treatment of decompensated heart failure.
Our results demonstrate Cinepazide has definite effectiveness in the treatment of heart failure and can alleviate symptoms including dyspnea, enhance the myocardial function and improve hemodynamics. As compared to Dobutamine, Cinepazide has a low incidence of side effects and favorable safety and patients are tolerable to Cinepazide treatment.
References
- Zhang L, Huang DF. Pharmacology and clinical application of Cinepazide maleate, a novel drug for cardiovascular diseases. J Chin Clin Med Res 2002.10(4): 7002-7003.
- Jordon JE, Zhao Z, Sato H. Agent s to improve cardiovascular circulation and cardiac metabolism of cinepazide. Circulation 1995. 92 (suppl 1): 520-524
- Gronstein BN. Adenosine, enendogenous ant inflammatory agent. J Appl Physiol 1994. 76(1): 5-13
- McMurray JJ, Pfeffer MA. Heart failure. Lancet 2005. 365: 1877-1889.
- Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002. 106: 3068-3072.
- Nieminen MS, Böhm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, Hasin Y, Lopez-Sendon J, Mebazaa A, Metra M, Rhodes A, Swedberg K, Priori SG, Garcia MA, Blanc JJ, Budaj A, Cowie MR, Dean V, Deckers J, Burgos EF, Lekakis J, Lindahl B, Mazzotta G, Morais J, Oto A, Smiseth OA, Garcia MA, Dickstein K, Albuquerque A, Conthe P, Crespo-Leiro M, Ferrari R, Follath F, Gavazzi A, Janssens U, Komajda M, Morais J, Moreno R, Singer M, Singh S, Tendera M, Thygesen K. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J 2005. 26: 384-416.
- Sharma M, Teerlink JR. A rational approach for the treatment of acute heart failure: current strategies and future options. Curr Opin Cardiol 2004. 19: 254-263.
- Dec GW. Acute decompensated heart failure: the shrinking role of inotropic therapy. J Am Coll Cardiol 2005. 46: 65-67.
- Deng YH, Dong XG, Hao YT. Prognostic potential of B-type natriuretic peptide and quality of life in patients with chronic heart failure South China J Cardiovasc Dis 2005. 11(1): 49-53.
- Xu JZ and Shi ZW. Brain natriuretic peptide and heart failure. Prevention and Treatment of Cardio-Cerebral- Vascular Disease 2003. 3(5): 5-56, 63.
- Dong HC, Guo YJ, Li L. The relationship between Btype natriuretic peptide, N terminal pro-B-type natriurefic peptide levels and cardiac function in heart failure patients. Lab Med 2006. 21(1): 58-60.
- Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA. Rapid measurement of B - type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002. 347: 161-167.
- Emiroglu Y, Kargin R, Kargin F, Akcakoyun M, Pala S, Mutlu H, Akcay M, Aung SM, Baran R, Ozdemir N. BNP levels in patients with long-term exposure to biomass fuel and its relation to right ventricular function. Pulm Pharmacol Ther 2010. 23(5): 420-424.