ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 7
Clinical analysis of patients diagnosed with hematopoietic malignancy superimposed with solid tumor
1Department of Haematology, the First People’s Hospital of Changzhou, Third Affiliated Hospital of Suzhou University, 185 Juqian Street, Changzhou, Jiangsu, P.R.China
2Department of Haematology, Children's Hospital Affiliated to Suzhou University, 185 Juqian Street, Changzhou, Jiangsu, P.R.China
#These authors contributed equally to this work
- *Corresponding Author:
- Weiying Gu
Department of Haematology
The First People’s Hospital of Changzhou
Third Affiliated Hospital of Suzhou University
P.R.China
- Xiaobao Xie
Department of Haematology
The First People’s Hospital of Changzhou
Third Affiliated Hospital of Suzhou University`
P.R.China
Accepted on December 2, 2016
Objective: The occurrence of superimposed malignancies, which has been receiving increased attention, has been recognized as a severe side effect following successful malignancy therapy. The purpose of the study is to analyse the clinical features and etiological characteristics of superimposed malignancies in our hospital.
Material and Methods: In the last 10 years at our hospital, 40 patients have been diagnosed with hematopoietic malignancy superimposed with solid tumors. Their clinical characteristics and therapies were summarized. Cytogenetic analyses were performed using quinacrine fluorescence and trypsin- Giemsa banding techniques. Overall survival rates were estimated using the Kaplan-Meier method.
Results: Twenty-four patients developed solid tumors first and then secondary hematopoietic malignancy, 13 patients developed hematopoietic malignancy first and then secondary solid tumor, and 3 patients developed 2 malignancies at the same time. Here, we report that the recurring balanced translocations and primary chemotherapy were found in most of the patients first diagnosed with hematopoietic malignancies, and these patients had significant differences when compared with the patients first diagnosed with solid tumors (P=0.0134 and P=0.013, respectively). Moreover, the patients who were first diagnosed with hematopoietic malignancy had shorter survival times than those first diagnosed with solid tumors (P=0.026).
Conclusion: These data confirm and extend previous studies of clinical characteristics, treatments, cytogenetic and prognosis findings in superimposed malignancies.
Keywords
Hematopoietic malignancy, Superimposition, Solid tumor.
Abbreviations
t-MN: therapy-related Myeloid Neoplasm; t-MDS: therapyrelated Myelodysplastic Syndrome; MM: Multiple Myeloma; CML: Chronic Myeloid Leukaemia; CLL: Chronic Lymphocytic Leukaemia; NHL: Non-Hodgkin's Lymphoma; APL: Acute Promyelocytic Leukaemia.
Introduction
With improvements in malignancy therapy, the survival time of patients has been prolonged. However, it is noteworthy that the incidence of secondary malignancies has been gradually rising, along with the successful treatment of first malignancy. Secondary malignancy, for which the possibility of it being a metastatic tumor has been ruled out, is distinct from the original disease, and it presents an independent picture of malignancy [1,2]. The latency time between first malignancy diagnosis and secondary malignancy diagnosis ranges from several months to decades. It has been reported that the latency period may depend on the cumulative dose or dose intensity of the previous cytotoxic or other specific therapy agents [3].
Therapy-related hematopoietic malignancies occurring after treatment with ionizing radiation or chemotherapy of solid tumors have been reported frequently [4-8]. In terms of cytogenetic characteristics and clinical behaviours, they are distinct from their de novo corresponding diseases. They are usually resistant to conventional chemotherapy and have a poorer prognosis. Meanwhile, with the development of novel targeted therapies and improvements in hematopoietic stem cell transplantation, the overall survival time of hematopoietic malignancy patients has been extended. Then, increasingly more secondary solid tumors have been diagnosed after first hematopoietic malignancy. Furthermore, the rare cases in which solid tumors and hematopoietic malignancies occur at the same time should also be noted.
In recent years, most studies have focused on therapy-related myeloid neoplasm (t-MN, including therapy-related Myelodysplastic Syndrome (t-MDS)) and therapy-related Acute Myeloid Leukaemia (t-AML), which occurs after radiotherapy or chemotherapy for solid tumor. Studies of secondary primary solid tumors have been relatively less reported. Furthermore, the studies of superimposed malignancies of china are rarely reported. The clinical features and etiological characteristics of these two disease situations have been rarely compared or analysed. Herein, we summarize the general information, chromosome karyotype and prognosis of the patients who underwent hematopoietic malignancy superimposed with solid tumor at our hospital during the past ten years, and we discuss what conclusions can be drawn for tumor therapy.
Materials and Methods
Patients
Patient samples were obtained with approval from the ethical committee at the First People's Hospital of Changzhou, Jiangsu province, China, and informed consent was obtained. From 2006 to 2015, after clinical, morphologic and genetic reviews in our department, 40 consecutive patients were confirmed to have been diagnosed with superimposed malignancies, including one case of hematopoietic malignancy. The latency interval was defined as starting with the diagnosis of the first malignancy and ending with the diagnosis of the secondary malignancy. When evaluating the latency interval and survival, and we ruled out the 3 patients who developed 2 malignancies at the same time; therefore, we analysed only 37 patients.
Malignancy diagnosis and treatment
Most of the 40 patients had been treated for their first malignancy at our hospital. All hematopoietic malignancies were diagnosed from marrow aspirates or marrow biopsy sections or lymph node biopsy. The first hematopoietic malignancies were primarily treated with chemotherapy; parts of the tumors were treated with immunomodulatory therapy or ionizing radiation in addition. All solid tumors were diagnosed through pathology and clinical manifestation. The first solid tumors were treated with surgical resection, ionizing radiation, chemotherapy, immunomodulatory therapy, or combination therapy, according to the type and stage of the disease. Treatments administered to patients after the development of secondary malignancies were individualized, ranging from supportive care only to intensive chemotherapy. If one malignancy was relatively stable, the treatment emphasis was on the other malignancy. If two malignancies occurred at the same time and the staging was similar, then the treatment was performed considering both malignancies, and the purpose of treatment was to prolong the patients’ overall survival as long as possible. Clinical data and details were collected from a review of each patient’s medical history. Patients were followed up until death or through October 2015.
Chemotherapy agents were classified by their mechanism of action. Alkylating agents include cyclophosphamide, chlorambucil, hydroxycarbamide, and oxaliplatin, etc. Topoisomerase 2 inhibitors include etoposide (VP16), daunorubicin, idamycin, epirubicin, pirarubicin, mitoxantrone, and irinotecan, etc. Antimetabolites include fluorouracil (5FU), xeloda, cytarabine, calcium folinate, hydroxyurea, and azathioprine, etc. Antitubulin agents include vincristine, vinorelbine, paclitaxel, and docetaxel, etc.
Cytogenetic analysis
Cytogenetic analyses were performed on bone marrow cells from aspirates obtained at the time of diagnosis using quinacrine fluorescence and trypsin-Giemsa banding techniques. Metaphase cell estimates were obtained from direct preparations and from 24 or 48 h non-irritant cultures. Cytogenetic abnormalities are described following the criteria of the International System for Human Cytogenetic Nomenclature [9].
Statistical methods
All statistical analyses were performed with SPSS 18.0 software. Qualitative variables were analysed with chi-squared tests or the Fisher’s exact test. Continuous variables were compared using Student’s t test or the Mann-Whitney U test. Survival rates were estimated using the Kaplan-Meier method. Overall survival was the interval between the diagnosis date and death. All statistical tests were 2 tailed, and P values less than 0.05 were considered to be statistically significant.
Results
Clinical characteristics
The clinical characteristics of the 40 superimposed malignancy patients are shown in Table 1. The series includes 18 women and 22 men. The median patient age at the time of diagnosis with a secondary malignancy was 64 years (range, 39-83 years). The distribution of first-diagnosed malignancies was as follows: 24 patients first developed solid tumors, 13 patients first developed hematopoietic malignancies, while 3 patients developed 2 malignancies at the same time. The hematopoietic malignancies that were first diagnoses involved MDS or AML (6 patients), multiple myeloma (MM, 4 patients), chronic myeloid leukaemia (CML, 1 patient), Chronic Lymphocytic Leukaemia (CLL, 1 patient) and Non-Hodgkin's Lymphoma (NHL, 1 patient). The solid tumors that were first diagnoses involved colon malignancy (8 patients), stomach malignancy (6 patients), breast malignancy (3 patients), lung malignancy (2 patients), oesophagus malignancy (1 patient), nasopharynx malignancy (1 patient), tongue malignancy (1 patient), prostate malignancy (1 patient) and renal malignancy (1 patient), in whom most were digestive tract tumors. The secondary hematopoietic malignancies involved MDS or AML (12 patients), NHL (5 patients), MM (2 patients), acute lymphoblastic leukaemia (ALL, 1 patient), CML (1 patient), CLL (1 patient), Chronic Myelomonocytic Leukaemia (CMML, 1 patient), and Plasma Cell Leukaemia (PCL, 1 patient). The secondary solid tumors involved stomach malignancy (7 patients), lung malignancy (3 patients), colon malignancy (2 patients), and cervix malignancy (1 patient).
| Primary diagnosis | No. of patients | Average age | Male | Female |
|---|---|---|---|---|
| Hematopoietic malignancy | 10 | 66.6 | 8 | 5 |
| MDS/AML(non APL) | 2 | 74 | 2 | 0 |
| APL | 4 | 57.75 | 1 | 3 |
| MM | 4 | 65.5 | 3 | 1 |
| Others | 3 | 75 | 2 | 1 |
| Solid tumor | 24 | 62.3 | 13 | 11 |
| colon malignancy | 8 | 60.75 | 2 | 6 |
| Stomach malignancy | 6 | 69.83 | 6 | 0 |
| Breast malignancy | 3 | 53.33 | 0 | 3 |
| Lung malignancy | 2 | 62 | 2 | 0 |
| Others | 5 | 63.4 | 3 | 2 |
| Two malignancies at the same time | 3 | 62.3 | 1 | 2 |
| Total | 40 | 63.7 | 22 | 18 |
Table 1. The clinical characteristics of superimposed malignancies patients.
Table 1 lists the average age and gender distribution at the time of the secondary malignancy diagnosis. In the group with hematopoietic malignancy as the first diagnosis, the average patient age was 66.6 years at the time the secondary solid tumor was diagnosed. In the group with solid tumor as the first diagnosis, the average age at the time the secondary hematopoietic malignancy was diagnosed was 62.3 years (P=0.5138, compared with the first diagnosed hematopoietic malignancy group). In the group with hematopoietic malignancy as the first diagnosis, there were 8 males and 5 females. Most of the patients who were first diagnosed with Acute Promyelocytic Leukaemia (APL) were men. In contrast, most of the patients who were first diagnosed with MM were women. In the group with solid tumor as the first diagnosis, there were 13 males and 11 females. Note that most of the patients who were first diagnosed with colon malignancies were women, while most of the patients who were first diagnosed with stomach malignancies were men.
Latency intervals
As shown in Table 2, the latency intervals between two types of diagnosed malignancies were estimated. The median latency intervals for the 37 patients was 57 months (IQR, 3-312 months), which ruled out the 3 patients who developed 2 malignancies at the same time. The latency intervals varied in the different malignancy categories. The median latency intervals of the 13 patients who developed secondary solid tumors after being first diagnosed with hematopoietic malignancies was 48 months (range, 7-108 months). The 24 patients who developed secondary hematopoietic malignancies after first being diagnosed with solid tumors had median latency intervals of 60 months (range, 3-312 months). The patients who were first diagnosed with hematopoietic malignancies tended to have shorter latency periods than the patients first diagnosed with solid tumors (48 months vs. 60 months), although this difference was not significant (P=0.4441). Meanwhile, when considering the age stratification, no significant differences in latency intervals were observed between younger and older superimposed malignancy patients (Table 3).
| Groups | No.of patients | Latency, months | P# | |
|---|---|---|---|---|
| Median | IQR | |||
| Hematopoietic malignancy→ Solid tumor | 13 | 48 | 7-84 | 0.4441 |
| Solid tumor →Hematopoietic malignancy | 24 | 60 | 3-312 | |
Note: #Mann Whitney test
Table 2. The latency intervals of superimposed malignancies patients.
| Age | No. of patients | Latency, months | P# | |
|---|---|---|---|---|
| Median | IQR | |||
| <55 | 11 | 48 | 3-168 | |
| 55-65 | 8 | 60 | 8-312 | 0.4133 |
| 65-75 | 8 | 75 | 30-204 | 0.1151 |
| 75 | 11 | 60 | 8-108 | 0.3558 |
Note: #Mann Whitney test, compared with<55 years group.
Table 3. The latency intervals of superimposed malignancies patients who stratified according to age.
Chromosome karyotype
Table 4 summarizes the clonal cytogenetic abnormalities of the 40 patients with superimposed malignancies. All patients underwent chromosome karyotype analysis at the time of hematopoietic malignancy diagnosis. This analysis was based on the hematopoietic malignancy diagnosis and primary therapy. In the group with hematopoietic malignancy as the first diagnosis, 3 of 13 patients had normal chromosome karyotypes, 2 patients had chromosome 5 and 7 abnormalities, and 8 patients had recurring balanced translocations, including t (15; 17), t (9; 22), t (14; 16), t (8; 21), inv (16), etc., while no patients were diagnosed with other clonal abnormalities. In the secondary hematopoietic malignancy group, 14 of 24 patients had normal chromosome karyotypes, 2 patients had chromosome 5 and 7 abnormalities, 5 patients had recurring balanced translocations, and 3 patients had with other clonal abnormalities. A significant difference was found between these two groups (P=0.0407) after comparing the chromosome karyotypes.
| Karyotype | No (%) |
|---|---|
| Hematopoietic malignancy→Solid tumor | 13 |
| Normal karyotype | 3(23.1%) |
| Clonal abnormalities of chromosome 5, 7, or both(±other abnormalities) | 2(15.4%) |
| Recurring balanced rearrangements | 8(61.5%) |
| Other clonal abnormalities | 0 |
| Solid tumor → Hematopoietic malignancy | 24 |
| Normal karyotype | 14(58.4%) |
| Clonal abnormalities of chromosome 5, 7, or both(±other abnormalities) | 2(8.3%) |
| Recurring balanced rearrangements | 5(20.8%) |
| Other clonal abnormalities | 3(12.5%) |
Table 4. The chromosome karyotype of superimposed malignancies patients.
The incidence of chromosome abnormality in the patients diagnosed with hematopoietic malignancy first was higher than in the patients diagnosed with secondary hematopoietic malignancy (10/13 vs. 10/24). The chromosome karyotype distribution differed between the different superimposed malignancies groups. The clonal abnormalities of chromosomes 5 and 7 were more popular in the group with hematopoietic malignancy diagnosed first than in the secondary hematopoietic malignancy group (15.4% vs. 8.3%). Recurring balanced translocations were observed in 8 patients in the secondary solid tumor group (61.5%), as well as in 6 patients with secondary hematopoietic malignancy (25%), and this difference was significant (P=0.0134).
Primary therapy and secondary malignancy
Table 5 shows the treatment for first malignancy in 2 different superimposed malignancies groups. Among the 13 patients who were diagnosed with hematopoietic malignancy first and then developed solid tumors, most underwent primary treatment with chemotherapy (12 of 13 patients, 92.3%). Among the 24 patients who first developed solid tumors and then developed hematopoietic malignancies, 12 patients (50%) underwent surgery resection, 11 patients (45.8%) underwent chemotherapy, and 6 patients (25%) underwent radiation therapy. A significant difference was observed between these two superimposed malignancies groups (P=0.0013) compared with the primary therapy group.
| First diagnosis | No. patients | CT | RT | Surgery | P# |
|---|---|---|---|---|---|
| Hematopoietic malignancy | 13 | 12(92.3%) | 0(0%) | 0(0%) | 0.0013 |
| Solid tumor | 24 | 11(45.8%) | 6(25%) | 12(50%) |
Note:#Chi-square test. In first diagnosis of hematopoietic malignancy group, one patient had no above treatment. In first diagnosis of solid tumor group, some patients had multiple treatments.
Table 5. The first therapy of superimposed malignancies patients.
Table 6 shows the specific drug therapy between two superimposed malignancies groups. In the first hematopoietic malignancy group, 6 patients (46.1%) received alkylators, 9 patients (69.2%) received topoisomerase 2 inhibitors, 5 patients (38.5%) received antimetabolites, and 2 patients (15.4%) received anti-tubulin. In the secondary hematopoietic malignancy group, 11 patients (45.8%) received alkylators, 7 patients (29.2%) received topoisomerase 2 inhibitors, 7 patients (29.2%) received antimetabolites, and 4 patients (16.7%) received anti-tubulin. There was no significant difference between these two superimposed malignancy groups in terms of specific drug usage (P=0.6163).
| Primary therapeutic agent | Hematopoietic malignancy-Solid tumor | Solidtumor-hematopoietic malignancy |
|---|---|---|
| Alkylator | 6/13(46.1%) | 11/24(45.8%) |
| Topoisomerase 2 inhibitor | 9/13(69.2%) | 7/24(29.2%) |
| Anti-metabolite | 5/13(38.5%) | 7/24(29.2%) |
| Anti-tubulin | 2/13(15.4%) | 4/24(16.7%) |
Table 6. The drug therapy of superimposed malignancies patients.
Survival
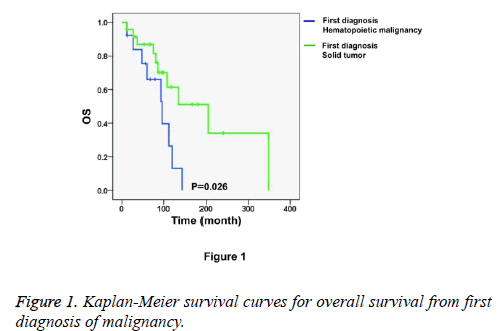
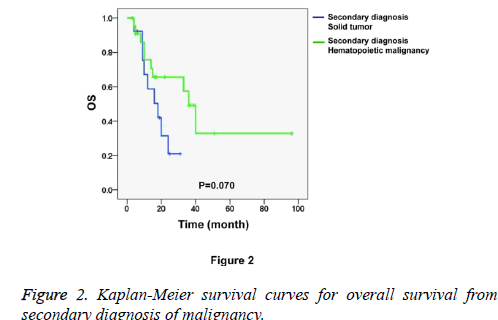
Figure 1 shows the Kaplan-Meier survival curves for overall survival from first diagnosis of malignancy. We found that the patients who were first diagnosed with hematopoietic malignancies had worse prognoses than the patients who were first diagnosed with solid tumors (P=0.026). The median time from the first diagnosis of hematopoietic malignancy (n=13) to death was 96.0 months, with a 95% Confidence Interval (CI) of 51.4 to 140.6 months. The median time from the first diagnosis of solid tumor (n=24) to death was 204.0 months, with a 95% CI of 88.8 to 319.2 months. Nevertheless, the secondary diagnosed malignancy showed a mild influence on the prognosis because the overall survival from the secondary diagnosis of malignancy showed no significant between-group differences (Figure 2, P=0.070). The median time from the secondary diagnosis of solid tumor (n=13, first diagnosis of hematopoietic malignancy) to death was 18.0 months, with a 95% CI of 8.0 to 28.0 months. The median time from secondary diagnosis of hematopoietic malignancy (n=24, first diagnosis of solid tumor) to death was 36.0 months, with a 95% CI of 28.9 to 43.1 months.
Discussion
Advances in malignancy therapy and supportive care measures have led to substantial improvements in long-term survival for malignancy patients. However, one of the most severe side effects following successful malignancy therapy is the occurrence of secondary malignancy. Secondary hematopoietic malignancy was the first reported carcinogenic effect of malignancy treatment. At present, secondary solid tumors have become more recognized and reported more frequently. Treatment of a pre-existing disease using chemotherapy, ionizing radiation therapy, surgery, or a combination of these modalities may lead to another destructive therapy-related secondary malignancy (solid tumor or hematopoietic malignancy) [10-13]. Chemotherapeutic agents or ionizing radiation may induce malignancy in the same manner that they cause DNA damage, which further results in mutations and translocations through DNA double-strand breaks and loss of DNA mismatch repair elements, with consequential genomic instability [14,15].
In our research, chemotherapy was performed in the vast majority of patients after the diagnosis of the first hematopoietic malignancy, but fewer than half of the patients accepted chemotherapy after first being diagnosed with a solid tumor. Thus, in theory, the secondary solid tumors should have a higher incidence than the secondary hematopoietic malignancies after the first treatment. Previous studies have reported that the incidence of secondary hematopoietic malignancies following the treatment of first solid tumors was much higher than that of secondary solid tumors following the treatment of first hematopoietic malignancies [16,17]. In our research, we analysed both secondary hematopoietic malignancy and secondary solid tumor cases in our hospital in the last decade. The distribution of patients also confirmed the conclusions of the aforementioned previous study. This phenomenon may be explained by the fact that the secondary malignancies reflect not only the late effects of first malignancy therapy but also the influence of the etiological factors that were shared with the first malignancy, such as environmental exposures, tobacco and alcoholic abuse, immune function, diet and hormonal status [18]. This phenomenon may also result from the relatively poor prognosis and short overall survival of patients with the first hematopoietic malignancy, which led to early deaths in the patients before the secondary solid tumor development. Here, there was a significant difference in the karyotype distribution between these two superimposed malignancies groups. Among the 13 patients with hematopoietic malignancy diagnosed first, most were found to have recurring balanced translocations. In the group with hematopoietic malignancy as a secondary diagnosis, the karyotypes of most patients were normal. This phenomenon suggests that the recurring balanced translocations in the hematopoietic malignancy patients might be related to the relatively higher incidence of secondary solid tumor development. We observed that most patients in the group with hematopoietic malignancy diagnosed first received chemotherapy, while in the other group; chemotherapy was performed in less than half of the patients. This result indicated that the carcinogenic potential of chemotherapy for the primary hematopoietic malignancy may be related to the development of the secondary solid tumor. Meanwhile, in the group with solid tumors as the primary diagnosis, many patients were not treated with chemotherapy or radiotherapy. The risk factors for the occurrence of secondary hematopoietic malignancy may be common etiological factors, including immune abnormalities, environmental exposure and chromosome aberration. Most importantly, we found a significant difference in the prognoses between these two superimposed malignancies groups. By determining the time interval between the first diagnosis and death, we observed that the patients who were first diagnosed with hematopoietic malignancy had generally shorter survival times than those first diagnosed with solid tumors. Many pathogenic factors may lead to this result. If a patient was first diagnosed with a hematopoietic malignancy, there was also a long period of bone marrow hematopoietic abnormalities with more complications (including infection, anaemia or haemorrhage). Such patients needed to use more antibiotics and have more blood-product transfusions, which may have increased the incidence of heart failure, lung and brain infarction, iron overload, etc. Moreover, the vast majority of the patients first diagnosed with hematopoietic malignancies were treated with combined chemotherapy, which can lead to multiple organs damage from the side effects. With the increased courses of chemotherapy, the bone marrow hematopoietic potential worsened, which may play a key role in the overall survival differences among patients with superimposed malignancies.
In conclusion, to treat and prevent the development of secondary superimposed malignancy, alleviation or cure of the first malignancy is the first clinical consideration. In addition, the alkylators should be used as little as possible. When the first malignancy occurs, particularly after treatment, a dynamic inspection and follow-up are important. When anaemia, bleeding, local neoplasm, or organ function deficiency occurs during the follow-up for malignancy, we should not assume that these conditions are caused by the first malignancy. It is possible that a secondary malignancy had already been formed at the time of the first malignancy. Early diagnosis and treatment can significantly improve patient prognosis and survival. Extensive studies should be conducted to clarify the underlying mechanism of secondary malignancies and to prolong the overall survival of patients diagnosed with hematopoietic malignancy superimposed with a solid tumor.
Financial Disclosure
The authors have no financial relationship to disclose.
Conflict of Interest
No conflicts of interest exist.
References
- Allan JM, Travis LB. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer 2005; 5: 943-955.
- Lene SG, Medeiros BC, Henrik S. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol 2015; 33: 3641-3649.
- Smith SM, Beau MML, Dezheng H. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukaemia: the University of Chicago series. Blood 2003; 102: 43-52.
- Thirman MJ, Larson RA. Therapy-related myeloid leukemia. Hematol Oncol Clin North Am 1996; 10: 293-320.
- Sill H, Olipitz W, Zebisch A, Schulz E, Wolfler A. Therapy-related myeloid neoplasms: pathobiology and clinical characteristics. Br J Pharmacol 2011; 162: 792-805.
- Bueso-Ramos CE, Kanagal-Shamanna R, Routbort MJ, Hanson CA. Therapy-related myeloid neoplasms. Am J Clin Pathol 2015; 144: 207-218.
- Kamihara Y, Sato Y, Takada K, Okagawa Y, Iyama S. Therapy-related myelodysplastic syndrome as a late adverse event of definitive chemoradiotherapy for esophageal and oropharyngeal cancer. Nihon Shokakibyo Gakkai Zasshi 2015; 112: 1664-1673.
- Bhatia S. Therapy-related myelodysplasia and acute myeloid leukemia. Semin Oncol 2013; 40: 666-675.
- Simons A, Shaffer LG, Hastings RJ. Cytogenetic nomenclature: changes in the ISCN 2013 compared to the 2009 edition. Cytogen Genom Res 2013; 141: 1-6.
- Larson RA, Le Beau MM. Prognosis and therapy when acute promyelocytic leukemia and other good risk acute myeloid leukemias occur as a therapy-related myeloid neoplasm. Mediterr J Hematol Infect Dis 2011; 3: e2011032.
- Adewuyi SA, Musa H, Samaila MO, Ogunrinde GO, Ameh EA. Pattern of paediatric solid cancers seen in radiotherapy and oncology department, Ahmadu Bello University Teaching Hospital, Zaria-Nigeria. Niger Postgrad Med J 2013; 20: 120-124.
- Lindahl LM, Fenger-Gron M, Iversen L. Secondary cancers, comorbidities and mortality associated with nitrogen mustard therapy in patients with mycosis fungoides: a 30-year population-based cohort study. Br J Dermatol 2014; 170: 699-704.
- Yuanlin XU, Wang H, Qian Z. Clinical analysis of non-hodgkins lymphoma associated with secondary malignant neoplasm. Chin J Clin Oncol 2012.
- Allan JM, Travis LB. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer 2005; 5: 943-955.
- Seedhouse C, Russell N. Advances in the understanding of susceptibility to treatment-related acute myeloid leukaemia. Br J Haematol 2007; 137: 513-529.
- Nadia H, Marie Cecile LD, Akhtar S. Role of radiotherapy and chemotherapy in the risk of secondary leukaemia after a solid tumour in childhood. Eu J Cancer 2006; 42: 2757-2764.
- Cheuk DK, Shek TW, Chan GC, Lau YL, Ha SY. Parotid acinar cell carcinoma in a long-term survivor of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 2008; 50: 636-639.
- Travis LB. Therapy-associated solid tumors. Acta Oncol 2002; 41: 323-333.