Keywords |
| Piper wallichii (Miq.) Hand.-Mazz; Chemical constituent; Tca83; Telomerase |
Introduction |
| As a plant in the genus Piper of the family Piperaceae with
vine as the medicinal part, Piper wallichii (Miq.) Hand.-Mazz.
has wind-cold dispelling, waist and knee strengthening and
kidney-yang invigorating functions, which is traditionally used
for rheumatic arthralgia, lumbocrural pain, etc. [1]. Piper
wallichii (Miq.) Hand.-Mazz. contains a variety of chemical
constituents, including lignins, amide alkaloids, organic acids,
sterols, etc. [2-4]. According to current findings, Piper
wallichii (Miq.) Hand.-Mazz. possesses multiple activities,
such as hepatoprotective, antioxidant, vasodilator,
antiarrhythmic and anticancer effects [5-7]. |
| To further reveal the material basis for its efficacy and
facilitate its development and utilization, we separated the
chemical constituents of Piper wallichii (Miq.) Hand.-Mazz.
extract to obtain five compounds, which were identified as:
futoenone (1), futoquinol (2), isofutoquinol A (3), futoamide
(4) and dihydropiperlonguminine (5). Degree of change in
telomerase activity is associated with cellular senescence,
immortalization and carcinogenesis; enhancement of
telomerase activity is an important aspect of malignant cellular
transformation [8-10]. According to statistics, over 85% of
malignant tumors have telomerase activity. This paper studies
the effect of Piper wallichii (Miq.) Hand.-Mazz. extract on
telomerase activity, and explores its antitumor mechanisms. |
Instruments and Materials |
| AVANCE 600 NMR spectrometer (TMS as internal standard,
Bruker); API4000 LC-MS system (Applied Biosystems);
Sephadex LH-20 gel (GE); ODS-A reversed phase silica gel
(YMC), silica gel (Qingdao Haiyang Chemical Plant); reagents
(AR, Nanjing Chengci Chemicals Co., Ltd.). Medicinal
material was purchased from Hebei Anguo Medicinal Material
Company, which was identified by Professor Fang Wenqi at
the China Medicine University as the dried vine of the
piperaceous plant Piper wallichii (Miq.) Hand.-Mazz. The
collection date is 2015.06.20, collector is Ting HUYAN,
Specimen number is 201506-28. |
| Piper wallichii (Miq.) Hand.-Mazz. extract (ethyl acetate
fraction) was self-prepared, and stored below -20, which was
diluted to the desired concentrations before use. TRAP-ELISA
reagents: telomerase detection kit S7750-KIT was purchased
from Chemicon, USA. SYBR Green I nucleic acid gel stain
was purchased from invitrogen, USA. Bradford working
solution and serum albumin used for protein determination
were purchased from Sigma, USA. |
Methods |
| Extraction and separation |
| 10 kg of Piper wallichii (Miq.) Hand.-Mazz. medicinal
material was extracted under reflux with an 8-fold amount of water for 2 h three times, and then the extracts were
concentrated into a gummy extract. A portion of the gummy
extract was dissolved in water, and extracted sequentially with
petroleum ether, ethyl acetate and n-butanol to give respective
fractions. Afterwards, the ethyl acetate fraction was separated
by chromatography on repeated positive phase silica gel,
reversed phase silica gel and Sephadex LH-20 columns and
recrystallized to give five compounds. |
| Cell cultivation |
| Human tongue squamous cell carcinoma Tca83 cells were from
the Stomatological Laboratory, Chengdu University of TCM,
which were cultured in an incubator at 37, 5% CO2 with
saturated humidity with 10% FBS-containing RPMI 1640
medium. |
| MTT assay of cell proliferation inhibition |
| Cell proliferative activity was determined by MTT assay. 180
μl of single cell suspension of logarithmic phase Tca83 cells
was seeded in each well of 96-well plates at 1×104 cells/well.
Meanwhile, control wells containing blank medium only were
set up. Each well was added with 20 μl of Piper wallichii (Miq.) Hand.-Mazz. extract until final concentrations of 0.200,
0.100, 0.050, 0.025 g/L were achieved. Each concentration had
three replicate wells. Meanwhile, a group of wells was added
with 20 μl of blank medium only to serve as a control. 24, 48,
72 h after medication, respectively, 5 g/L MTT solution was
added at 20 μl/well; and 4 h later, 200 μl of DMSO was added.
Then, absorbances of samples were measured at A490 nm with
a microplate reader, and averaged by group. Finally, growth
inhibition rate was calculated according to the following
formula: growth inhibition rate=[1- (treatment - background) /
(control - background)] × 100%. |
| Telomerase activity detection |
| PCR-ELISA assay: Telomeric repeats (TTAGGG) were added
to the 3' end of biotin-labeled synthetic PI(TS) primer by
telomerase. Through repeated PCR amplification of specific
product between PI(TS) and P(RP) primers, PCR product
containing telomerase-specific 6-nucleotide was generated.
After denaturation, the PCR product was hybridized to the
probe for digoxin-labeled telomerase-specific repetitive
sequence, and the resulting hybrid was immobilized onto
biotin-affinity protein-coated microplate via biotin-labeled
probes. Afterwards, the immobilized PCR product was
detected with a peroxidase-conjugated antibody (Anti-DIGPOD).
Finally, peroxidase substrate TMB produced colored
reaction to visualize the probes. |
| Telomerase extraction: 106 cells were placed into a sterile
reaction tube containing 200 μl of chilled lysate. After ice
bathing for 30 min, the cells were centrifuged at 12,000 g at 4
for 20 min. Then, supernatant was carefully removed into a
DEPC-treated tube, protein content in the extraction liquid was
determined by Bradford assay, and each specimen was adjusted for protein concentration to 1 g/L and cryopreserved below -80
for later use. |
| TRAP assay: 50 μl reaction system included dNTP, Taq
polymerase, internal standard primer, biotinylated primer (TS)
5'-AATCCGTCGAGCACAGTT-3' and biotinylated primer
(RP). Primers were extended with 1 μl of extraction liquid: 1
cycle at 30 for 30 min; PCR amplification: 33 cycles at 94 for
30 s and at 55 for 30 s. |
| Electrophoresis: 10% non-denaturing polyacrylamide gel and
SYBR Green I nucleic acid gel stain were used.
Electrophoresis conditions: vertical electrophoresis tank; 0.5 ×
TBE (Tris-Borate-EDTA) buffer; gel electrophoresis apparatus;
voltage of 400 V; 90 min. Gels were imaged with GDS-800 gel
imaging system. |
| Hybridization and ELISA procedures of PCR product: 5 μl
of PCR amplification product and 20 μl of denaturant were
incubated at room temperature for 10 min, added with 225 μl
of hybridizing buffer, and mix well. 100 μl of the mixture was
then placed onto avidin-coated microplates, and incubated in a
37, 300 r/min shaker for 2 h. After washing, 100 μl of anti-
DIG-POD was added for reaction at 18~22 for 30 mim.
Finally, 100 μl of POD substrate TMB was added for color
development for 10 min, and then the reaction was terminated
by adding stop buffer. Absorbance was measured at 450 nm
and 690 nm with a microplate reader, and A=A450-A690 value
was calculated for each sample. Absorbance difference ΔA was
judged as positive if higher than 0.150. ΔA=A sample - A
inactivated sample. |
| Statistical analysis |
| Experimental data obtained were expressed as x ± s, and all
data were statistically processed using SPSS 13.0 for windows
software. Differences between groups were compared by oneway
ANOVA, and P<0.05 was considered statistically
significant. |
Results |
Structure elucidation |
| Compound 1: white needle crystals (methanol). 1H-NMR (600
MHz, CDCl3) δ: 6.78 (1H, d, J = 7.8 Hz, H-17), 6.78 (1H, d,
J=1.8 Hz, H-14), 6.75 (1H, dd, J=7.8, 1.8 Hz, H-18), 6.02 (2H,
s, H-19), 5.87 (1H, s, H-2), 5.51 (1H, s, H-5), 5.09 (1H, m,
H-8), 3.72 (3H, s, OCH3), 2.59 (1H, dt, J=12.0, 6.0 Hz, H-10),
2.43 (1H, m, H-7β), 2.31 (1H, m, H-9β), 2.22 (1H, m, H-7α),
2.12 (1H, m, J=14.3, 6.6 Hz, H-11), 1.76 (1H, dd, J=14.4, 12.0
Hz, H-9α), 0.64 (3H, d, J = 6.0 Hz, 12-CH3); 13C-NMR (150
MHz, CDCl3) δ: 183.2 (C-3), 180.2 (C-1), 154.7 (C-4), 147.7
(C-15), 146.7 (C-16), 137.3 (C-13), 123.0 (C-18), 109.2
(C-17), 108.7 (C-14), 107.9 (C-2), 101.5 (C-5), 101.2 (C-19),
81.7 (C-8), 55.3 (C-7), 50.3 (-OMe), 46.5 (C-6), 45.6 (C-10),
43.7 (C-9), 38.1 (C-11), 14.6 (C-12). The above data were
consistent with the reports in literature [11], so compound 1
was identified as futoenone. |
| Compound 2: white crystals (methanol). 1H-NMR (600 MHz,
CDCl3) δ: 6.71 (1H, s, H-7'), 6.87~6.74 (3H, m, H-2', 5', 6'),
6.16 (1H, s, H-2), 5.99 (2H, s, -OCH2O-), 5.89 (1H, m, J = 15.6,
6.6 Hz, H-8), 5.89 (1H, s, H-5), 5.15 (2H, m, H-9), 3.81 (3H, s,
4-OCH3), 3.29 (3Hs, 3-OCH3), 3.16 (2H, m, H-7), 1.69 (3H, d,
J = 1.2 Hz, 8'-CH3); 13C-NMR (150 MHz, CDCl3) δ: 188.2
(C-6), 172.5 (C-4), 147.5 (C-3'), 147.3 (C-4'), 142.1 (C-2),
139.9 (C-1), 135.2 (C-8), 133.5 (C-8'), 131.7 (C-1'), 128.2
(C-7'), 123.2 (C-6'), 117.3 (C-9), 110.2 (C-2'), 109.1 (C-5'),
105.9 (C-5), 103.1 (-OCH2O-), 80.1 (C-3), 56.3 (4-OMe), 52.4
(3-OMe), 31.9 (C-7), 14.1 (8'-CH3). The above data were
consistent with the reports in literature [12], so compound 2
was identified as futoquinol. |
| Compound 3: colorless oil. 1H-NMR ( 600 MHz, CDCl3) δ:
6.64 (1H, d, J=7.8 Hz, H-5'), 6.42 (2H, m, H-2',6'), 6.12 (1H, m,
H-2″), 5.78 (2H, s, -OCH2O-), 5.31 (2H, m H-3″), 4.96 (1H, s,
H-4), 3.87 (1H, s, H-7), 3.57 (3H, s, 3-OMe), 3.41 (3H, s, 2-
OMe), 2.89 (1H, dd, J=14.4, 6.6 Hz, Ha-1″), 2.57 (1H, dd,
J=14.4, 7.8 Hz, Hb-1″), 2.29 (1H, s, H-1), 1.62 (3H, s, H-9); 13C-NMR (150 MHz, CDCl3) δ: 196.1 (C-5), 173.2 (C-3),
147.6 (C-3'), 146.3 (C-4'), 134.7 (C-2″), 133.2 (C-1'), 119.4
(C-6'), 118.7 (C-3″), 107.7 (C-5'), 106.8 (C-2'), 104.4 (C-4),
101.2 (-OCH2O-), 67.8 (C-2), 56.9 (2-OMe), 56.5 (3-OMe),
53.9 (C-6), 51.1 (C-7), 39.6 (C-1″), 36.3 (C-8), 35.8 (C-1),
12.9 (8-Me). The above data were basically consistent with the
reports in literature [13], so compound 3 was identified as
isofutoquinol A. |
| Compound 4: white crystals (methanol). Dark spots by 10%
phosphomolybdic acid ethanol solution, and under 254 nm UV
light. ESI-MS m/z: 302.5, [M+H]+. 1H-NMR ( 600 MHz,
CDCl3) δ: 6.92~6.75 (4H, m, H-3,2″,5″,6″), 6.34 (1H, d, J=15.6
Hz, H-7), 6.02 (1H, m, H-6), 5.92 (2H, s, -OCH2O-), 5.82 (1H,
d, J = 15.6 Hz, H-2), 5.43 (1H, brs, -NH), 3.17 (2H, t, J=7.2 Hz,
H-1'), 2.35 (4H, m, H-4,5), 1.82 (1H, m, H-2'), 0.96 (6H, d,
J=6.6 Hz, H-3'); 13C-NMR (150 MHz, CDCl3) δ: 166.5 (C-1),
147. 1 (C-3″), 147.7 (C-4″), 143.7 (C-3), 131.6 (C-1″), 132.1
(C-7), 127.0 (C-6), 124.3 (C-2), 120.4 (C-6″), 107.1 (C-5″),
105.9 (C-2″), 101.2 (-OCH2O-), 46.3 (C-1'), 32.1 (C-5), 31.7
(C-4), 28.9 (C-2'), 19.0 (C-3'). The above data were basically
consistent with the reports in literature [14], so compound 4
was identified as futoamide. |
| Compound 5: white crystals (methanol). Dark spots by 10%
phosphomolybdic acid ethanol solution, and under 254 nm UV
light. ESI-MS m/z: 276.2 [M+H]+. 1H-NMR (600 MHz,
CDCl3) δ: 6.79 (1H, m, H-3), 6.75 (1H, d, J=7.8 Hz, H-5″),
6.67 (1H, d, J=1.8 Hz, H-2″), 6.62 (1H, d, J=7.8 Hz, H-6″),
5.92 (2H, s, -OCH2O-), 5.76 (1H, d, J = 15.6 Hz, H-2), 5.45
(1H, brs, -NH), 3.14 (2H, t, J=7.8 Hz, H-1'), 2.69 (2H, t, J = 7.2
Hz, H-5), 2.45 (2H, m, H-4), 1.81 (1H, m, H-2'), 0.92 (6H, d,
J=6.6 Hz, 2'-Me); 13C-NMR (150 MHz, CDCl3) δ: 165.9
(C-1), 146.9 (C-3″), 145.5 (C-4″), 143.3 (C-3), 134.4 (C-1″),
124.2 (C-2), 121.2 (C-6″), 108.9 (C-5″), 108.6 (C-2″), 101.7 (-
OCH2O-), 46.6 (C-1'), 34.5 (C-5), 34.3 (C-4), 28.7 (C-2'), 21.0
(C-3'). Its 1H-NMR data were basically consistent with the
literature [15], so compound 5 was identified as
dihydropiperlonguminine. |
| Antiproliferative effect of Piper wallichii (Miq.)
Hand.-Mazz. extract on Tca83 cells |
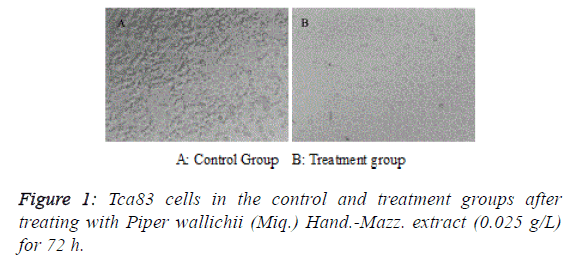
| After treating Tca83 cells with Piper wallichii (Miq.) Hand.-
Mazz. extract for 72 h, adherent cells in the treatment group
presented apparent apoptotic features such as shrinkage,
rounding and shedding under an inverted fluorescence
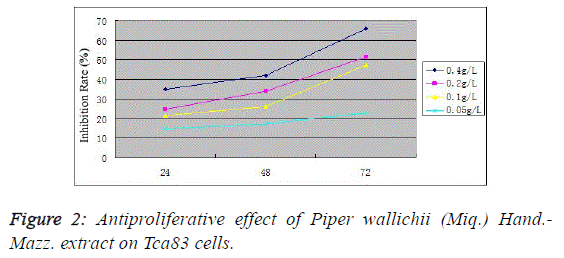
microscope (Figure 1). MTT assay found that the proliferation
inhibition rates in the 0.4, 0.2, 0.1 and 0.05 g/L Piper wallichii (Miq.) Hand.-Mazz. extract groups were (34.9 ± 4.1)%, (24.7 ±
3.8)%, (21.4 ± 2.1)% and (14.5 ± 2.9)%, respectively, at 24 h;
(42.1 ± 2.4)%, (33.7 ± 4.1)%, (25.9 ± 1.9)% and (17.2 ± 2.3)%,
respectively, at 48 h; and (65.9 ± 3.7)%, (51.4 ± 2.9)%, (47.1 ±
3.2)% and (22.9 ± 2.1)%, respectively, at 72 h. Compared with
the control group, the proliferation activity significantly
decreased in various experimental groups at corresponding
time periods (P<0.05). For the same concentration group,
antiproliferative action increased with prolonging time
(P<0.05), indicating the Tca83 cell proliferation inhibiting
effect of Piper wallichii (Miq.) Hand.-Mazz. extracts in vitro in
a time- and concentration-dependent manner (Figure 2). |
 |
 |
| Telomerase activity expression |
| TRAP assay of sample extract showed an internal standard
band at 36 bp, and appearance of bands at a 6 bp interval from
50 bp, forming alternately dark and bright ladder bands, which
indicated positive telomerase activity. Presence of internal
standard only would indicate negative telomerase activity. Gel
electrophoresis demonstrated that the alternately dark and
bright bands darkened with increasing drug concentration and
prolonging action time, especially for the 0.2 g/L group, where
72 h sample showed markedly darker bands than the positive
control. This suggested that YHL-TA had a certain inhibitory
effect on telomerase activity of Tca83 cells. |
| ELISA semi-quantification of telomerase activity |
| Expression of telomerase activity decreased gradually in the
control group, as well as in the 0.1 and 0.2 g/L Piper wallichii (Miq.) Hand.-Mazz. extract groups. Telomerase activity was
already inhibited for the 0.2 g/L Piper wallichii (Miq.) Hand.-
Mazz. extract group at 24 h; and at 72 h, telomerase activity was significantly lower for the Piper wallichii (Miq.) Hand.-
Mazz. extract groups than the control group (P<0.05). At the
same concentrations, enhanced inhibition of telomerase
activity was noted with prolonging action time (Table 1, Figure
3). |
Discussion |
| Telomerase is present in most tumor cells, while in normal
cells, its activity is undetectable. Telomerase activation is a
must way to cell immortalization, and immortalization is an
important step in malignant transformation of tumors.
Therefore, some scholars have proposed the telomerase theory
of carcinogenesis [16]. |
| Anti-tumor constituents from TCM extracts have been
receiving increasing attention. Recent studies have found that a
lot of chemical constituents from plants have anti-tumor
activities, which can be categorized into the following: lignins,
neoliginins, amide alkaloids, organic acids, sterols, etc. [17]
With the deepening of research on telomere and telomerase,
oncotherapy studies targeting the inhibition of telomerase
activity have been increasing, and it has been confirmed that
many natural herbal ingredients have an anti-telomerase action
[18]. Inhibition of telomerase activity by natural small
molecules is expected to become a more effective and safer
novel antineoplastic protocol since it differs from the ordinary
cytotoxic antitumor therapies. |
| Piper wallichii (Miq.) Hand.-Mazz. extract, which contains
active constituents of the plant, has hepatoprotective, analgetic,
sedative, immunoenhancing and antioxidant effects. Piper
wallichii (Miq.) Hand.-Mazz. has definite clinical efficacy, which is used in combination with other drugs in most cases.
Anticancer activity of Piper wallichii (Miq.) Hand.-Mazz.
extract has been scarcely studied. To this end, this study
reported the effects of Piper wallichii (Miq.) Hand.-Mazz.
extract on proliferation and telomerase activity of human
tongue squamous cell carcinoma Tca83 cells and its anticancer
mechanisms. |
Conclusion |
| Piper wallichii (Miq.) Hand.-Mazz. extract used in this
experiment is self-prepared by recrystallization technique
based on the systematic research of chemical constituents. The
extract mainly contains five compounds: futoenone (1),
futoquinol (2), isofutoquinol A (3), futoamide (4) and
dihydropiperlonguminine (5). |
| Results of this study show that the Piper wallichii (Miq.)
Hand.-Mazz. extract can inhibit the proliferation of tongue
carcinoma cells, and suppress telomerase activity with
increasing time and concentration. Thus, interruption of tongue
carcinoma genesis and progression is expected by inhibiting
telomerase activity to cause lost infinite proliferative capacity
of cells. For telomerase, this molecular biological target of
oncotherapy, Piper wallichii (Miq.) Hand.-Mazz. is a good
candidate drug. |
Acknowledgement |
| This work is supported in part by a grant from the National
Natural Science Foundation of China (NSFC, Grant No.
31500688), the China Postdoctoral Science Foundation (Grant
No. 2014M560804) and the Natural Science Foundation of
Shaanxi Province (Grant No. 2015JQ8307 and 2014JM1002). |
References |
- Editorial Committee of Flora of China, Chinese Academy of Sciences. Flora of China. Volume IIX, Fascicule 1. Beijing: Science Press 1982.
- Shi Y, Yang L, Zhao J, Shi Y, Qu Y, Zhu H, Wang D, Yang C, Li X, Xu M, Zhang Y. Chemical constituents from Piper wallichii. Natural Product Research 2015; 29: 1372-1375.
- Shi YN, Shi YM, Yang L, Li XC, Zhao JH, Qu Y, Zhu HT, Wang D, Cheng RR, Yang CR, Xu M, Zhang YJ. Lignans and aromatic glycosides from Piper wallichii and their antithrombotic activities. Journal of Ethnopharmacology 2015; 162: 87-96.
- Tamuly C, Hazarika M, Bora J, Gajurel PR. Antioxidant Activities and Phenolic Content of Piper wallichii (Miq.) Hand.-Mazz. International Journal of Food Properties 2014; 17: 309-320.
- Zhao GW, Xia W, Chen P, Han EJ, Xiang L. Study on the bioactive constituents of Piper wallichii. Zhong Yao Cai 2012; 35: 53-56.
- Li XF. Effects of flavone II of Piper wallichii on the isolated rabbit heart. Zhong Yao Tong Bao 1985; 10: 37-39.
- Fang JY, Thomson C. Hydrogen-bonding effects, electrostatic potential, and the antitumor activity of flavones acetic acid and related compounds. II. Ab initio studies on the second stable conformations. International Journal of Quantum Chemistry 1996; 60: 897-909.
- Shay JW, Wright WE. The reactivation of telomerase activity in cancer progression. Trends Genet 1996; 12: 129-131.
- Wright WE, Brasiskyte D, Piatyszek MA, Shay JW. Experimental elongation of telomeres extends lifespan of immortal and normal cell hybrids. EMBO J 1996; 15: 1734-1741.
- Kim NW, Piatyszek MA, Prouse KR. Specific association of human telomerase activity with immortal cells and cancer. Science 1994; 266: 2011-2015.
- Woods MC, Ogiso MI, Kurabayashi M, Mishima H. The NMR spectra of futoenone and derivatives: partial decoupling as an aid in the assigment of complex spectra, and further observations of overhauser effects. Tetrahedron Letters 1968; 9: 2009-2014.
- Tyagi OD, Jensen S, Boll PM, Sharm NK, Bishta KS, Parmara VS. Lignans and neolignans from Piper schmidtii. Phytochemistry 1993; 32: 445-448.
- Ohba S, Yamashita H, Saito Y, Shizuri Y, Nakamura K, Yamamura S. Structure of isofutoquinol A. Acta Crystallographica. Section C 1987; 43: 172-173.
- Das B, Kashinatham A. Futoamide from Piper longum. FITOTERAPIA 1998; 69: 548-552.
- Dwuma-Badu D, Ayim JSK, Dabr TT, ElSohly HN, ElSohly MA, Knapp JE, Slatkin DJ, SchiffJr PL. Δβ-dihydropiperlonguminine, a new amide from Piper guineense. Phytochemistry 1976; 15: 822-823.
- Hiyama E, Yokoyama T, Tatsumoto N, Hiyama K, Imamura Y, Murakami Y, Kodama T, Piatyszek MA, Shay JW, Matsuura Y. Telomerase activity in gastric cancer. Cancer Research 1995; 55: 3258-3262.
- Ji ZX, Zhao B, Li WJ, Li FF, Li ZX, Cui C, Shen WX, Gong J, Ni SF. Overview of pharmacological research on Piper wallichii (Miq.) Hand-Mazz. Journal of Anhui Agricultural Sciences 2012; 18: 9663-9665.
- Naasani I, Seimiva H, Tsuruo T. Telomerase inhibition,telomere shortening, and senescence of cancer cells by tea catechins. Biochem Biophys Res Commun 1998; 249: 391-396.
|

