ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 4
Assessment of quality of life in Chinese patients with thyroid associated orbitopathy
1Department of Medical Social Work, Dongying People's Hospital, Dongying, Shandong, China
2Health Management Division, Dongying People's Hospital, Dongying, Shandong, China
3Department of Health, Dongying People's Hospital, Dongying, Shandong, China
4Department of Clinical Support, Dongying People's Hospital, Dongying, Shandong, China
5Department of Health Materials Management, Dongying People's Hospital, Dongying, Shandong, China
6Medical Department, Dongying People's Hospital, Dongying, Shandong, China
#These authors contribute to this study equally
- *Corresponding Author:
- Lifang Han
Department of Medical Social Work
Dongying People's Hospital, China
Accepted date: September 23, 2016
Objective: To examine the quality of life (QOL) of Chinese people with thyroid-associated orbitopathy by employing TAO-QOL Questionnaire, to test compliance of questionnaire, and to evaluate the correlation between TAO-QOL and various classifications of TAO.
Design: Prospective and cross-sectional.
Participants: Total 182 patients with TAO and Grave’s disease enrolled in the present study.
Methodology: The original TAO-QOL was translated into Chinese language for the present study. In our study the findings were compared using various clinical severity grading systems such as CASs, modified NOSPECS score, VISA classification, EUGOGO classification, and Gorman diplopia scale.
Results: Clinical scores demonstrating inflammation and strabismus in study subjects with TAO are positively associated with overall and visual function-linked QOL (Spearman coefficient 0.03-0.38) p<0.05). Clinical measures related with appearance are positively associated with appearance-associated QOL (Spearman coefficient 0.25-0.26, p<0.05). The multivariate analysis of the present study revealed, motility disorder of VISA classification and age, soft tissue inflammation, motility disorder of modified NOSPECS exhibited positive correlation with overall and function-related QOL. Similarly, soft-tissue inflammation, proptosis, gender of modified NOSPECS, and appearance of VISA classification had correlation with appearance-related QOL. Moreover, rationality of TAO-QOL was verified adequately based on the findings of patient surveys and correlation between the subscales of TAO-QOL.
Conclusion: TAO-QOL displayed substantial compliance with various objective clinical measures of TAO. TAO-QOL was a lucid and convenient tool for quick assessment of QOL in daily outpatient wards, which is an easily translatable into several languages and extensively applicable to various inhabitants of different geographical regions.
Keywords
Quality of life, Thyroid-associated orbitopathy, China
Introduction
Thyroid-associated ophthalmopathy (TAO) also termed as thyroid-associated orbitopathy, is a long-term inflammatory and auto immune ailment, and generally results in several complex symptoms and impairments. The alterations resulting from TAO can show a strong negative influence on patient’s daily life [1]. Terwee et al. did the pioneering effort in creating the QOL questionnaire (Graves’ Ophthalmopathy-QOL [GOQOL]) specific for TAO. The original GO-QOL consisted of 16 queries on appearance and visual functioning [2]. In addition to Terwee et al., several other studies, witnessed that the GO-QOL was effective and dependable to examine QOL of patients with TAO [3-6]. Moreover, GO-QOL was reliable in assessing the correlations with clinical activity and severity of TAO, in among various geographical populations and in several language settings [3-6]. Further, the European Group on Graves' Orbitopathy (EUGOGO) also endorsed GO-QOL, for employing it as a tool for examining the clinical development to the interventional clinical studies [7]. By using the Korean version of GO-QOL, Choi et al. reported the QOLTAO in Korean population [5]. In this study, GO-QOL scores of Korean population were substantially correlated with disease rigorousness and activity calculated by clinical activity scores (CASs) and NOSPECS (no signs or symptoms, only signs, soft tissue, proptosis, extraocular muscle, cornea, sight loss) [5].
Even though GO-QOL has been widely employed to examine QOL of TAO, it could be of tedious, time taking and often it may not be feasible to practice in day today clinics, due to its lengthiness and larger number queries. To make the assessment simpler, recently Fayers and Dolman have created an easy 3- item questionnaire in English termed as TAO-QOL [8]. Additionally, TAO-QOL has been found to be quick, easy to fill, score and analyze the findings [8]. Moreover, TAO-QOL was reasonably correlated with VISA (vision loss (opticneuropathy); inflammation; strabismus/ motility; appearance/exposure) classification scores. The objective of the present study was to examine the impaired QOL of Chinese patients with TAO, by employing the TAO-QOL questionnaire and assess correlations between TAO-QOL measurements and other several well-known scales of disease severity and activity.
Materials and Methods
Prior to starting the present study, we have gotten approval from the ethics committee of Dongying People's Hospital to proceed. Informed consent was obtained from all the study subjects prior to the study subject’s enrolment in the study. In the present study, a total of 182 subjects with TAO and Grave’s disease were enrolled. The present study was prospective, cross-sectional, consisting of Chinese patients who were followed up from Aug-2012 to Dec-2015. Subjects who had other eye diseases that may affect the QOL were omitted. All the study subjects received the TAO-QOL self-administered questionnaires at the outpatient ward, department of ophthalmology, and filled in them prior to medical examination. Clinical data obtained are displayed in Table 1.
The data pertaining to thyroid-stimulating hormone receptor autoantibodies (TSHR Ab), consisting of thyroid bindinginhibiting immunoglobulin and thyroid-stimulating immunoglobulin (TSI), were collected within a month of the study inception. In the present study, an experienced ophthalmologist performed all the clinical observations and examinations. The disease-specific TAO-QOL questionnaire created by Fayers and Dolman [8] was altered to the Chinese language using forward and backward translation [9]. The TAO-QOL contained 3 single-item questions: how TAO affected on the patient’s appearance, visual functioning, and overall QOL in the patient’s daily routine life. The TAO queries were scored from 0 to 10, where 0=TAO did not interfere with QOL and 10=TAO completely interfered with QOL. After finishing with the QOL questionnaire, subjects were enquired about whether any of the questions were puzzling or hard to grade, and whether the questions were related and inclusive.
Soft-tissue inflammation and activity of TAO were assessed by the CASs ranging from 0 to 7 [10]. TAO was defined as an active state when CAS was more than 3. The severity of TAO was estimated according to the following classification scores [11-13]. The VISA classification was grouped as follows: vision (0 or 3 points), inflammation (0-8 points), S1 (diplopia), S2 (motility restriction), and appearance/exposure (0-3 points). The total of the VISA grouping scores varied from 0 to 20 (most severe). In the present study, we have employed the modified NOSPECS score, which was used in a similar study by Choi et al. [5,12,14]. The modified NOSPECS was classified as: lid retraction (0 or 1 point), soft-tissue inflammation, proptosis, site difference, extraocular muscle involvement (0-3 points), corneal defects (0 or 1 point), and optic nerve compression (0 or 3 points). The overall modified NOSPECS score varied from 0 to 17 (utmost severe). EUGOGO severity assessment divided subjects with TAO into 3 groups: mild, moderate-to-severe, and sight-threatening groups. Motility disorders were grouped according to the Gorman diplopia scale (0-3 points) [15].
All the data were evaluated using SPSS version 20.0 (SPSS, Chicago, Ill.). Employing two-sided p values and a p (<0.05) was taken as statistically significant. We considered TAO-QOL scores as a dependent variable and the following as independent variables: bilaterality, age, sex, history of smoking, duration of TAO and Graves’ disease, activity and severity scores of TAO, and abnormality of TSI and TSHR Ab. To analyze relationships between each subscale of TAO-QOL and continuous variables, we calculated the Spearman correlation coefficient. According to Cohen et al.’s recommendations, correlations were considered low (r<0.2), moderate (0.2<r <0.5), or high (r>0.5) [16]. The relationship between TAO-QOL and categorical variables was calculated by the Mann-Whitney U test or Kruskal-Wallis test.
To examine which constituent of the clinical severity score was an important predictor of QOL scores, we have executed multiple linear regression analysis (stepwise regression) with possible confounders (age and gender). To assess floor and ceiling effects, we calculated the fraction of patients scoring 0 (minimum value) and 10 (maximum value) for each of the items of TED-QOL. According to Bradley et al., [17] more than 15% and more than 30% of maximum value were considered significant and substantial ceiling effects, respectively. Significant and substantial floor effects were defined in the same way. Content rationality dealt with whether TAO-QOL covered all health-related qualities of life relevant for the intended purpose. This was evaluated through patient interviews about the coverage and relevance of TAO-QOL. Convergent validity and discriminant validity, procedures to measure the construct validity, were assessed by correlation between the subscales of TAO-QOL.
Results
In the present study, we have interviewed total 182 subjects with regard to the every point of TAO-QOL. On an average, it took around 2 minutes per subject for discussion and completion of filling up the TAO-QOL. We have indicated the demographic data and clinical features of study subjects in the Table 1. The mean CAS is 1.78 and 28 (15.3%) subjects showed active TAO. Nine study subjects (4.9%) showed optic neuropathy during the survey. Beyond 50% of the patients with TAO with diplopia showed moderately severe TAO, based on the EUGOGO severity scale. Most of the study subjects exhibited moderately severe QOL score as gauged by the TAOQOL questionnaire (Table 2). Mean scores for overall, function and appearance are, 7.71, 7.32 and 7.78 respectively. The scores of overall (36.8%) and appearance (44.5%) demonstrated a significant ceiling effect. Moreover, the visual function-associated QOL also showed a marked (28.5%) ceiling effect. However, the results didn’t depict significant floor effect.
| Characteristics | Values |
|---|---|
| Mean age ± SD (range), y | 42.1 ± 14.7 (20-70) |
| Sex (F/M), n (%) | 131 (71.9%)/51 (28.0%) |
| Duration of TED ± SD (range), mo | 18.9 ± 16.4 (1-144) |
| Duration of GD ± SD (range), mo | 33.2 ± 28.2 (1-180) |
| Bilateral unilateral manifestation, n (%) | 150 (82.4%)/32 (17.5%) |
| Other autoimmune disease, n (%) | 14 (7.6%) |
| History of smoking, n (%) | 45 (24.7%) |
| Family history of thyroid disease, n (%) | 31 (17.0%) |
| Best corrected visual acuity ± SD (range) | |
| Right eye | 0.8 ± 0.3 (0.03-1.1) |
| Left eye | 0.8 ± 0.3 (0.03-1.1) |
| Lid traction from normal position of lid ± SD (in patients with lid retraction, n=80 (range) | |
| Upper lid (from 2 mm below limbus) | 2.2 ± 0.8 (1-4) |
| Lower lid (from limbus) | 1.2 ± 0.5 (0.6-3) |
| Total | 2.0 ± 1.0 (0.5-6) |
| Exophthalmos ± SD (range), mm | |
| Right eye | 17.6 ± 2.7 (11-24) |
| Left eye | 17.4 ± 2.6 (11-23) |
| Site difference | 1 ± 1 (0-5) |
| Treatment of GD, n (%) | |
| Antithyroid drugs | 136 (74.7%) |
| Radioiodine therapy | 15 (8.2%) |
| Thyroidectomy | 31 (17.0%) |
| Treatment of TAO, n (%) | |
| Steroid (oral/intravenous) | 114 (62.6%)/49(26.9%) |
| Local triamcinolone injection | 42 (23.0%) |
| Radiotherapy | 7 (3.8%) |
| Decompression | 28 (15.3%) |
| Eye muscle surgery | 0 |
| Eye lid surgery | 14 (7.6%) |
| Clinical feature of patients with TAO | |
| Mean CAS ± SD (range) | 1.78 ± 1.3(0-7) |
| Mean modified NOSPECS score SD (range) | 5.1 ± 2.7 |
| Active TED (CAS ≥ 4) | 28 (15.3%) |
| Optic nerve involvement, n (%) | 11 (6.1%) |
| Severity by EUGOGO classification, n (%) | |
| Mild | 58 (31.8%) |
| Moderately severe | 126 (69.2%) |
| Sight-threatening | 9 (4.9%) |
| Current Gorman score of diplopia score n (%) | |
| 0 | 89 (48.9%) |
| 1 | 60 (32.9%) |
| 3 | 25 (13.7%) |
| 4 | 8 (4.3%) |
| Mean VISA score ± SD (range) | 5.5 ± 2.8 (1-14) |
| Mean TSI ± SD (range), SRR% | 432.7 ± 219.7 (39.7-890.7) |
| Positive, n (%) | 154 (84.6%) |
| Mean TSHR Ab ± SD (range), IU/ml | 10.5 ± 7.8 (0-38) |
| Positive, n (%) | 132 (73.0%) |
| Thyroid function, n (%) | |
| Euthyroid | 118 (64.8%) |
| Hyperthyroid | 58 (31.8%) |
| Hypothyroid | 6 (3.2%) |
| TAO: Thyroid-Associated Orbitopathy; GD: Graves’ Disease; CAS: Clinical Activity Score; NOSPECS: No Signs or Symptoms, Only signs, Soft tissue, Proptosis, Extraocular muscle, Cornea, Sight loss; EUGOGO: European Group on Graves’ Orbitopathy; VISA: Vision loss (optic neuropathy), Inflammation, Strabismus/motility, Appearance/exposure; TSI: Thyroid-Stimulating Immunoglobulin; SRR: Specimen-to-Reference Ratio; TSHR Ab: Thyroid-Stimulating Hormone Receptor Autoantibodies | |
Table 1. Clinical characteristic features of study subjects with thyroid associated oribitopathy at the time of survey.
| Subscale | Range | Mean ± SD | Patients at Ceiling, n (%) | Patients at Floor, n (%) |
|---|---|---|---|---|
| Overall | 0-10 | 7.71 ± 2.1 | 67 (36.8%) | 7 (3.8%) |
| Function | 0-10 | 7.32 ± 2.3 | 52 (28.5%) | 5 (2.7%) |
| Appearance | 0-10 | 7.78 ± 2.7 | 82 (44.5%) | 5 (2.7%) |
Table 2. Thyroid eye disease quality-of-life questionnaire scores.
During the survey, major part of the study subjects said that the questionnaire was lucid and easily understandable. In addition, the study subjects also said that the questionnaire was exhaustive and covered all the aspects of TAO. Seven (3.8%) subjects said it was slightly tough to scale each item separately. Moreover, 4 (2.1%) subjects stated a requirement for some extra items related to ocular uneasiness, such as ophthalmic irritation, tearing and photophobia. The convergent and discriminant rationality were evaluated by association between the subscales of TAO-QOL. The TAO-QOL questionnaire exhibited a good convergent rationality as there were positive correlations between overall and visual function-associated QOL (r=0.81, p=0.001) and overall and appearance associated QOL (r=0.69, p=0.001). Even though the correlation between visual function and appearance was good, the discriminant validity of TAO-QOL was good, i.e weaker relation (r=0.55, p=0.001). Moreover, the content rationality of TAO-QOL demonstrated adequate through patient surveys, and TAO-QOL was indirectly assessed as valid based on the results of correlations between questionnaires and respective clinical measure.
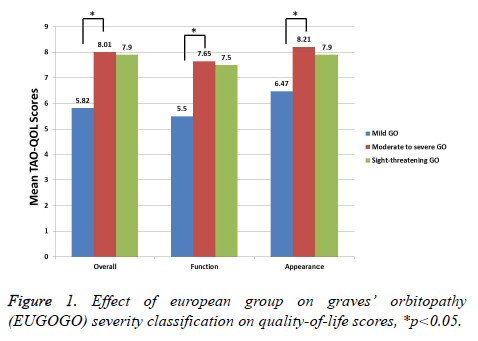
We have shown the correlations between the scales of TAOQOL and clinical scores in Table 3. Clinical measures showing the inflammation grade of TAO, such as CAS, I of VISA grading system, and soft inflammation of NOSPECS, were reasonably correlated with overall (all p<0.01) and visual function-associated QOL (all p<0.05). The Clinical measure demonstrating strabismus of TAO, such as S1 and S2 of VISA grading system, extraocular muscle association of NOSPECS, and Gorman scores, were moderately positively related with overall (all p<0.05) and visual function-associated QOL (all p<0.05). As anticipated, clinical measures related with appearance, such as A of VISA grading system and proptosis of NOSPECS, were moderately correlated with appearancerelated QOL (all p<0.05). Moreover, total measures of VISA grading system and modified NOPSECS were moderately correlated with all items of TED-QOL (all p>0.05). All the measures of TAO-QOL were substantially dissimilar between mild, moderate and severe TAO (p<0.05). Conversely, there was no statistical variance between sight-threatening TAO and others, as there is only small number of subjects are with sight threatening TAO (p>0.05) (Figure 1). Moreover, there is statically no difference between the TAO-QOL measures such as sex, bilaterality, lid retraction, smoking behaviour, disease activity, dysthyroid optic neuropathy, and abnormal TSI or TSHR Ab (p>0.05).
| TAO-QOL | ||||||
|---|---|---|---|---|---|---|
| Overall | Function | Appearance | ||||
| Spearman Coefficient (95% CI) |
p | Spearman Coefficient (95% CI) |
p | Spearman Coefficient (95% CI) |
p | |
| CAS scores | 0.26 (0.07~0.43)* | 0.008* | 0.22 (0.05-0.41)* | 0.018* | 0.17 (-0.01 to 0.33) | 0.61 |
| VISA scores | ||||||
| I | 0.27 (0.07-0.43)* | 0.007* | 0.23 (0.05-0.41)* | 0.019* | 0.19 (-0.02 to 0.38) | 0.052 |
| S1 | 0.19 (0.03-0.38)* | 0.048* | 0.21 (0.05-40)* | 0.021* | 0.11 (-0.12 to 0.31) | 0.347 |
| S2 | 0.31 (0.12-0.49)* | 0.003* | 0.31 (0.14-0.47)* | 0.003* | 0.18 (-0.02 to 0.35) | 0.108 |
| A | 0.12 (-0.06 to 0.32) | 0.182 | 0.12 (-006 to 0.33) | 0.178 | 0.26 (0.08-0.39) | 0.014 |
| Total | 0.37 (0.18-0.53)* | <0.001* | 0.41 (0.21-0.56)* | <0.001* | 0.28 (0.14-0.49)* | 0.005* |
| NOSPECS | ||||||
| Soft-tissue inflammation | 0.368 (0.17-0.51)* | <0.001* | 0.358 (0.18-0.49)* | <0.001* | 0.258 (0.07-0.42)* | 0.01* |
| Proptosis | 0.129 (-0.08 to 0.31) | 0.218 | 0.114 (-0.09 to 0.29) | 0.286 | 0.257 (0.08-0.41)* | 0.013* |
| Site difference | 0.029 (-0.14 to 0.26) | 0.798 | 0.02 (-0.18 to 0.25) | 0.837 | -0.098 (-0.29 to 0.10) | 0.32 |
| EOM | 0.338 (0.13-0.49)* | 0.001* | 0.331 (0.12-049)* | 0.001* | 0.172 (-0.02 to 0.37)* | 0.097* |
| Total | 0.34 (0.14-0.49)* | 0.001* | 0.33 (0.13-0.50)* | 0.001* | 0.25 (0.08-0.43)* | 0.013* |
| Gorman scores | 0.21 (0.04-0.39)* | 0.04* | 0.23 (0.06-0.42)* | 0.021 | 0.006 (-0.14 to 0.25) | 0.537 |
| TAO: Thyroid-Associated Orbitopathy; QOL: Quality Of Life; CAS: Clinical Activity Score; VISA, Vision loss (optic neuropathy), Inflammation, Strabismus/motility, Appearance/exposure; I: Inflammation; S1-diplopia; S2-motility restriction; A: Appearance; NOSECS: No signs or symptoms, Only signs, Soft tissue, Proptosis, Extraocular muscle, Cornea, Sight loss; EOM: Extraocular Muscle Involvement,*p<0.05. | ||||||
Table 3. Correlations between thyroid eye disease quality-of-life questionnaire and clinical severity scores.
We have executed multiple linear regression analysis with for each component of clinical measure (Table 4). The modified NOSPECS score, overall and functional scores of QOL were positively correlated with scores of soft-tissue inflammation (regression coefficient β=1.012 ± 0.312 and 1.078 ± 0.312, respectively; p<0.01) and motility disorder (β=0.978 ± 0.251 and 0.896 ± 0.25; p<0.01), and weakly positively correlated with age (β=0.043 ± 0.021 and 0.03 ± 0.02; p<0.05). Appearance scores of QOL were correlated with measures of soft-tissue inflammation (β=0.859 ± 0.349) and proptosis (β=0.786 ± 0.312), and showed higher scores in female patients (B=1.47 ± 0.572; p<0.05). Similarly, in cases of VISA measure, overall and function scores of QOL were positively correlated with motility disorder (S2, β=1.119 ± 0.277 and 0.821 ± 0.263; p<0.01), and weakly positively correlated with age (β=0.043 ± 0.021 and 0.03 ± 0.02; p<0.05). Appearance scores of QOL were correlated with appearance (A, β=0.935 ± 0.306) and showed higher scores in females (β=1.218 ± 0.567; p<0.05).
| β Coefficient ± SE | 95% CI of β | p | ||
|---|---|---|---|---|
| Overall QOL | NOSPECS | |||
| Age | 0.043 ± 0.021 | 0.010-0.086 | 0.012 | |
| Soft-tissue inflammation | 1.012 ± 0.312 | 0.356-1.643 | 0.004 | |
| EOM | 0.978 ± 0.251 | 0.469-1.507 | <0.001 | |
| VISA | ||||
| Age | 0.043 ± 0.02 | 0.005-0.081 | 0.024 | |
| S2 | 1.119 ± 0.277 | 0.543-1.677 | <0.001 | |
| Function QOL | NOSPECS | |||
| Age | 0.03 ± 0.02 | 0.001-0.069 | 0.042 | |
| Soft-tissue inflammation | 1.078 ± 0.312 | 0.412-1.742 | 0.02 | |
| EOM | 0.896 ± 0.25 | 0.35-1.431 | 0.003 | |
| VISA | ||||
| S2 | 0.821 ± 0.263 | 0.289-1.373 | 0.003 | |
| Appearance QOL | NOSPECS | |||
| Sex | 1.47 ± 0.572 | 0.416-2.801 | 0.007 | |
| Soft-tissue inflammation | 0.859 ± 0.349 | 0.128-1.589 | 0.021 | |
| Proptosis | 0.786 ± 0.312 | 0.196-1.384 | 0.009 | |
| VISA | ||||
| Sex | 1.218 ± 0.567 | 0.089-2.481 | 0.035 | |
| A | 0.935 ± 0.306 | 0.327-1.498 | 0.005 | |
| SE: Standard Error; QOL: Quality Of Life; EOM: Extra-Ocular Muscle Involvement; S2: Motility Restriction; A: Appearance. | ||||
Table 4. Association of thyroid eye disease quality-of-life with clinical severity scores and the possible confounders (age and sex) by multiple linear regression analyses.
Discussion
In the present study, we have surveyed patients with TAO and Graves’ disease by using a questionnaire, which was originally prepared in Korean and eventually translated into Chinese. The observations were compared with clinical severity scores such as CAS, VISA, modified NOSPECS, Gorman diplopia scale and EUGOGO classification. In the results of the present study, we have witnessed that the clinical scores demonstrating inflammation and strabismus in TAO subjects were correlated with overall and visual function-associated QOL. Similarly, clinical measures related with appearance were correlated with appearance-associated QOL. Moreover, age, soft tissue inflammation, motility disorder of modified NOSPECS, and motility disorder of VISA grading system exhibited correlation with overall and function-related QOL. Further, gender, softtissue inflammation, proptosis of modified NOSPECS, and appearance of VISA grading demonstrated correlation with appearance-related QOL. In conclusion, TAO-QOL demonstrated substantial correlations with various clinical parameters of TAO.
The association between GO-QOL assessments for several populations and numerous objective clinical scores of TAO were examined in earlier studies [2,4-6]. In those studies, the visual activity and appearance measures of GO-QOL exhibited a fine correlation with motility measure and proptosis scales respectively. Further, Fayers et al. stated that ED-QOL and GO-QOL measure were reasonably correlated with VISA measures [8]. Similarly, in a Korean study overall and function-associated TAO-QOL was moderately associated with the grades of inflammation, diplopia and motility function in all clinical grading scores. Further, the grades linked to appearance in NOSPECS and VISA scales were associated only with QOL related to appearance. As TAO-QOL correlated with anticipated clinical measure and all types of grading systems, we consider that the present questionnaire could provide dependable QOL evidence, irrespective of evaluation methods.
In Fayers et al. study, there was no data regarding the mean and the range of TAO-QOL, but a substantial ceiling effect was seen for the function scale (19%) and a marked floor effect was also seen for the appearance scale (21%) [8]. In our study, although the clinical disease severity of study subjects looked alike Fayers et al. study, there was not a marked floor effect on any items of the Korean TAO-QOL. Moreover, substantial ceiling effects (27.8%-47.8%) were in seen in all the measures (Table 2). In this regard, some theories could clarify the probable reasons for such differences. A few social psychological investigators witnessed self-critical tendencies in self-evaluation and underestimation of daily life contentment among Asians who regarded themselves as comparatively worse compared with the Westerners [18]. In contrary, they found self-enhancement among the Westerners, who rated themselves as comparatively better than others.
An additional variance that may influence QOL is approach versus avoidance orientation. Asians who consider themselves as part of a group tend to focus on the negative consequences (avoidance), whereas Westerners consider themselves as an independent self-incline to focus on the positive consequences (approach) [19]. Similarly, another change may be in communication fashion. Asian cultures greatly value the harmony in interactive relationships and do not show interest in open expression of a full gamut of emotions consisting of negative and positive, but wish more delicate nonverbal communication [20]. People with TAO generally suffer from disfiguration like proptosis and lid retraction, and interferes with social communication. In other terms, these alterations appearance could perturb eye contact and blinking, which play a vital part in sustaining the flow of social communication, and the disfiguration can make patients unintentionally appear to present with facial expression related with anger, surprise, or fear [21]. Thus, it is not surprising that the Asians who incline indirect and nonverbal expression in human contact are more affected psychologically by TAO than the Westerners. These inter-cultural changes may have had more adverse influence on the QOL in Asians. In addition, psychological illness may continue and develop chronic, although severity of TAO may decline with passage of time and as a result of treatment [21]. Consequently, the lengthier the duration of TAO, the increased the TAO-QOL scores could be, compared with the expected results from clinical scores. This could explain why the TAOQOL of patients in this study with longer duration of TAO exhibited relatively higher scores than scores stated by Fayers and Dolman [8].
We can see a ceiling effect when the study subjects who notch the maximum value of test items cannot be assessed beyond the examined gauges because of the limited number of items to select [22]. It leads to constraint in the discriminative capability of the questionnaire to recognize deterioration in study subjects who already have a poor QOL. A floor effect is opposite to the ceiling effect, which prevents the questionnaire from recognizing improvement in subjects who already have good QOL. As there was a substantial ceiling effect of TAOQOL in our study, the questionnaire could be rigorous in detecting exacerbated change of QOL in relatively severe cases. Nonetheless, because of the floor effect of all the items of the questionnaire was rare, TAO-QOL may be employed as the tool for examining the improvement of QOL with treatment. GO-QOL was initially created in Dutch and translated to 8 different languages, and it has been extensively employed for several populations. There were some complications in cross-cultural adaptation with GO-QOL, particularly in terms of visual functioning. As GO-QOL contains questions about specific activities, the importance and meaning perceived by the patients could be dependent on the population features. For instance, limitations in bicycling are vital for Dutch people, but could be less vital for people in other countries. Furthermore, several items of GO-QOL could result in more missing answers than TED-QOL; 79%-85% of the patients completed all of the GO-QOL questionnaire, but missing response rates of some questions were up to 15% [4-6].
However, due to TAO-QOL contained only 3 questions, it was simpler to translate into many other languages, and crosscultural variations were lower than GO-QOL. Moreover, missing replies on TAO-QOL were rare, as 100% of the study subjects completed all the questions for TAO-QOL in both English and Chinese [8]. Additionally, in both Fayers study and in our present study exhibited that survey time duration for TAO-QOL was lesser than those for GO-QOL: less than 2 minutes compared to GO-QOL. Furthermore, interpretation would be much easy and faster for TAO-QOL because GOQOL has several item scales and it takes more time to convert numerical values and exclude missing values. The present study multiple limitations. First, we gathered data of study subjects in a tertiary care hospital of a single academic institution. The study subjects from other clinics could have had above-average disease severity. Second, this is crosssectional study and didn’t allow an examination of the responsiveness of alterations of TAO-QOL in TAO patients over time and with treatment. Third, as relatively a small number of study subjects (n=4 [2.1%]) had sight threatening TAO, the most severe, complication of TAO, these complications didn’t statistically influence TAO-QOL.
Conclusion
In conclusion, TAO-QOL had fine correlation with the clinical severity measurements similar with GO-QOL. It may be used in diverse geographical populations and in several languages and it would be convenient in objective clinical measurements. Furthermore, its usage would be more convenient in crosscultural translation with good correlation with specific clinical measurements of TAO. In addition, TAO-QOL is a swift and easy way to measure and very much convenient for data entry and data analysis. Due to these conveniences, TAO-QOL has become clinical more useful in rapid QOL assessment of population in a daily outpatient clinic. Further, using QOL could make us to take prompt decisions a better treatment modality. Lastly, including psychosocial supports by identifying psychosocial impairment and in measuring improvement of QOL after treatment would be useful in crafting new therapeutic modalities.
References
- Estcourt S, Quinn AG, Vaidya B. Quality of life in thyroid eye disease: impact of quality of care. Eur J Endocrinol 2011; 164: 649-55.
- Terwee CB. Development of a disease specific quality of life questionnaire for patients with Graves' ophthalmopathy: the GO-QOL. Br J Ophthalmol 1998; 82: 773-779.
- Terwee CB. Test-retest reliability of the GO-QOL: a disease-specific quality of life questionnaire for patients with Graves' ophthalmopathy. J Clin Epidemiol 1999; 52: 875-884.
- Ponto KA. Quality of life in a german graves orbitopathy population. Am J Ophthalmol 2011. 152: 483-490.
- Choi YJ. Assessing Graves' ophthalmopathy-specific quality of life in Korean patients. Eye (Lond) 2012; 26: 544-551.
- Park JJ. Assessing quality of life in Australian patients with Graves' ophthalmopathy. Br J Ophthalmol 2004; 88:75-78.
- Wiersinga WM. Clinical assessment of patients with Graves' orbitopathy: the European Group on Graves' Orbitopathy recommendations to generalists, specialists and clinical researchers. Eur J Endocrinol 2006; 155: 387-389.
- Fayers T, Dolman PJ. Validity and reliability of the TED-QOL: a new three-item questionnaire to assess quality of life in thyroid eye disease. Br J Ophthalmol 2011; 95: 1670-1674.
- Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol 1993; 46: 1417-1432.
- Mourits MP. Clinical criteria for the assessment of disease activity in Graves' ophthalmopathy: a novel approach. Br J Ophthalmol 1989; 73: 639-644.
- Dolman PJ, Rootman J. VISA Classification for Graves orbitopathy. Ophthal Plast Reconstr Surg 2006: 22: 319-324.
- Eckstein AK. Thyrotropin receptor autoantibodies are independent risk factors for Graves' ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab 2006; 91: 3464-3470.
- Bartalena L. Consensus statement of the European Group on Graves' orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol 2008; 158: 273-285.
- Son BJ, Lee SY, Yoon JS. Evaluation of thyroid eye disease: quality-of-life questionnaire (TED-QOL) in Korean patients. Can J Ophthalmol 2014; 49: 167-173.
- Gorman CA. Radiotherapy for Graves' ophthalmopathy: results at one year. Thyroid 2002; 12: 251-255.
- de Boer AG. Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual Life Res 2004; 13: 311-320.
- Bradley EA. Evaluation of the National Eye Institute visual function questionnaire in Graves' ophthalmopathy. Ophthalmology 2006; 113: 1450-1454.
- Diener E, Oishi S, Lucas RE. Personality, culture, and subjective well-being: emotional and cognitive evaluations of life. Annu Rev Psychol 2003; 54: 403-425.
- Lee AY, Aaker JL, Gardner WL. The pleasures and pains of distinct self-construals: the role of interdependence in regulatory focus. J Pers Soc Psychol 2000; 78: 1122-34.
- Nilchaikovit T, Hill JM, Holland JC. The effects of culture on illness behavior and medical care. Asian and American differences. Gen Hosp Psychiatry 1993; 15: 41-50.
- Coulter I. Psychological implications of Graves' orbitopathy. Eur J Endocrinol 2007; 157: 127-131.
- McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res 1995; 4: 293-307.