ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2016) Volume 27, Issue 3
Assessment of melatonin and cortisol levels in sickle cell anemia children: a single center based study.
Marwa M El-Sonbaty1,2, Mohammed AA Al Zolaly1, Shereen A El Tarhouny3,5*, Zakaria MH Al Hawsawi4
1Pediatric Department, College of Medicine, Taibah University, Madinah, KSA
2Department of Child Health, Medical Research Division, National Research Centre (Affiliation ID: 60014618), Dokki, Cairo, Egypt
3Medical Biochemistry Department, College of Medicine, Taibah University, Madinah, KSA
4Pediatric Department, Maternity and Children Hospital, Madinah, KSA
5Medical Biochemistry Departement, College of Medicine, Zagazig University, Egypt
- Corresponding Author:
- Shereen El Tarhouny
Medical Biochemistry Department College of Medicine Taibah University Saudi Arabia
Accepted date: February 25, 2016
Objective: To correlate serum levels of melatonin and cortisol in sickle cell anemia (SCA) with age, age at diagnosis, sex, anthropometry, vaso-occlusive crisis (VOC) and current treatment.
Methods: Twenty nine patients and 29 controls were recruited into this study. Melatonin and Cortisol assays were performed using commercially available ELISA kit. Statistical analysis was done to assess the difference in mean melatonin and cortisol levels and correlate these with age, age at diagnosis, sex, anthropometry, Vaso-Occlusive Crisis (VOC), current treatment.
Results: There was a significantly lower melatonin serum levels in SCA patients compared to controls (p<0.001). Melatonin was negatively correlated with age, vaso-occlusive crisis and cortisol (r=-0.02, p=0.9), (r=-0.13, p=0.5), (r=-0.28, p=0.15) respectively. However, melatonin levels were not correlated with other parameters including weight and height. There was slightly small difference in the mean levels of cortisol in patients compared to controls, but this difference was not statistically significant. Similarly, no correlation with age, weight, or height was noted. There was no difference in mean serum levels of melatonin and cortisol between males and females of SCA patients also, between that receiving HU when compared with that not receiving HU.
Conclusion: In the present work, we observed a significant decrease in melatonin level in SCA patients compared to controls. This highlights melatonin importance as a therapeutically agent for developing antioxidant defence. The use of melatonin will certainly help in decreasing oxidative damage and will ultimately results in detracting symptoms associated with SCA.
Keywords
Sickle cell anemia, Melatonin, Cortisol, Children.
Introduction
Sickle cell anemia (SCA) results from a mutation in the genetic code such that glutamic acid is replaced by valine in the globin chain of haemoglobin [1]. This substitution transforms normal adult hemoglobin (HbA) into sickle hemoglobin (HbS).When deoxygenated, HbS polymerizes, and when a critical amount of HbS polymer accumulates within a sickle erythrocyte, cellular injury occurs [2]. A sufficient number of damaged erythrocytes cause the phenotype of sickle cell disease (SCD), characterized by hemolytic anemia and vaso-occlusion [3,4].
Sickle cell anaemia is one of the diseases that exhibit circadian rhythm in their symptoms. Rhythmic leukocyte/endothelial cell interactions can contribute to obstructing blood flow in small caliber vessels by exacerbating leukocyte activation, prompting their interactions with other free-flowing blood components such as red blood cells (RBCs), and potentially causing thrombus formation and vascular infarction as observed in SCD [5], and sometimes leads to increase oxidative stress [6]. It has been proposed that melatonin has antioxidant properties [7-9] and increase in cortisol concentration has been associated with decrease in total antioxidant levels and low total antioxidant capacity is a common feature in all conditions where elevated cortisol concentrations are observed [10]. The importance of melatonin as an antioxidant is due to its capacity to cross biological barriers, which eases the removal of reactive oxygen species (ROS) in different biological compartments [11].
An improved understanding of the abnormal oxidative processes that occur in SCA has led to new insights into the action mechanisms of some currently accepted therapies and it has also suggested new therapies for this disease [12,13]. For this reason, antioxidant therapy is being a worthy, promising, and increasing goal for SCA treatment. In addition, one of the most appealing properties of melatonin which distinguishes it from most antioxidants is that its metabolites also have the ability to scavenge ROS.
Melatonin is an endogenously produced indoleamine secreted by the pineal gland. Melatonin secretion has a circadian rhythm and is influenced by environmental light. [14]. Melatonin has been found to directly modulate cortisol levels. Cortisol is another hormone which has circadian rhythm [15]. Normal individuals, without disease of the hypothalamo-pituitary adrenal axis, at midnight, have very low or undetectable cortisol levels that build up overnight to peak first thing in the morning. Cortisol levels then decline slowly throughout the day [16-18]. Therefore melatonin is a hormone that supports a healthy immune system, proper sleep and is the main hormone involved in synchronizing the daily cycle of light exposure and physical activity in the body. While cortisol is critical for maintaining energy and supporting a healthy immune system. Hence both are the major hormones of the circadian system.
Previous studies in rats and primates found that glucocorticoid measures decreased after melatonin [19,20], also melatonin attenuates corticosterone reactivity to an acute or chronic stressor [19]. In young humans, single or repeated administration of melatonin did not affect basal or pharmacologically stimulated cortisol secretion [21].
In the present study we aim to analyze the effect of melatonin and cortisol on the sickle cell anemia. In particular, we have to explore the relation between serum levels of melatonin and cortisol in SCA and correlate that with different disease parameters. Additionally, we aim to assess if SCA does affect interactions between these hormones.
Patients and Methods
This was a prospective case-control study conducted at the maternity and children hospital (MMCH), Al Madinah Almonowarah, Saudi Arabia, and at Pediatric and Medical Biochemistry Department, Taibah University, Al Madinah Almonowarah, Saudi Arabia. Children aged 6-16 years, were enrolled into this study. The study included children having sickle cell anaemia attending haematology clinic and agree to participate in the study. Sickle cell anaemia diagnosis was based on hemoglobin electrophoresis. The subjects were screened using a questionnaire and an interview. They were excluded if they were smokers, drinkers, or were taking medication known to affect melatonin production, such as β- adrenergic blockers. The patients qualified for the study gave their written informed consent, which was approved by the ethics committee of the Madinah Maternity and Children Hospital (MMCH) and Taibah University. Additionally, we recruited 29 apparently healthy children of the same age and socioeconomic standards as a control group.
Detailed history-taking and thorough clinical examinations were performed. At enrollment, the number of severe painful episodes in the preceding 12 months was recorded (frequency of vaso-occlusive crisis (VOC) per year), with a working definition of a vaso-occlusive crisis (VOC) as pain in the extremities, back, abdomen, chest, or head that led to an unscheduled clinic or emergency room visit and required hospitalization, and that could only be explained by SCD, with exclusion of any episode of pain that was treated entirely at home [22].
Biological samples
After informed consent signed by legal guardians, 3 mL of peripheral blood samples were collected through venipuncture around 7:30 AM.
Biochemical assay used to determine melatonin and cortisol levels in blood sample
Three mL of blood sample was collected from the antecubetal vein in a plain vacutainers, centrifuged at 3,000 g at 37°C for 20 minutes and the serum was separated and stored at –20°C until processing. A commercially available ELISA kit was used to quantify melatonin (CUSABIO BIOTECH CO catalog No. CSB-E08132h) and cortisol (R&D Systems, Minneapolis, USA catalog No.KGE008) levels in the serum samples using horseradish peroxidase detection in accordance with the manufacturer’s instructions. 100 μL of sample for melatonin and cortisol were used. Samples were assayed as duplicates. The minimum detection limit was less than 7.8 pg/ml and 0.071 ng/ml respectively. Results were finally obtained by subjecting the 96 well microtitreplate to the ELISA reader at 405 nm.
Statistical analysis
Patients’ data were analyzed using the Microsoft Excel and Statistical Package for Social Sciences (SPSS), version 16.0 for Windows. Quantitative variables were expressed by mean ± standard deviation (SD), and compared using Student’s t-test for unpaired samples and Spearman’s rank-order test was used for correlating quantitative variables. Qualitative variables were expressed as numbers (frequency) and percentages, and compared between groups using the chi-squared test. p-value was considered to be significant if <0.05.
Results
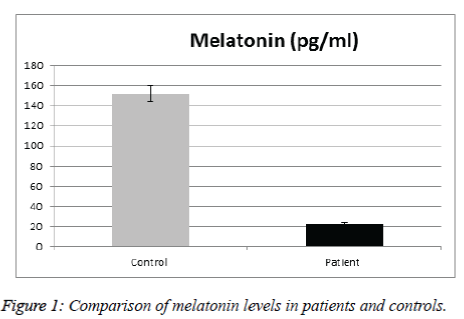
Table 1 illustrates a comparison of the clinic epidemiologic data between SCA patients and the control group. No significant difference between patients & controls regarding age, sex, anthropometric parameters, nationality, number of sibling & consanguinity. Mean values of melatonin was significantly lower in SCA patients than in control group (Figure 1).
| Parameter | Patients (n=29) | Controls (n=29) | P-value |
| Clinico-epidemiologic data | |||
| Age in years (mean ± SD) | 10.4±2.7 | 9.7±2 | NS |
| Sex (male/female) | 14/15,(48.3%; 51.7%) | 11/18,(37.9%; 62.1%) | NS |
| Weight in Kg (mean± SD) | 28.2±9 | 32.6±7.5 | NS |
| Height in cm (mean± SD) | 132.9±12.4 | 137.8±10.9 | NS |
| BMI(kg/m2) (mean±SD) | 15.9±2.4 | 16.9±1.7 | NS |
| Ethnicity (Saudi/non- Saudi) |
19/10 (65.5%; 34.5%) |
17/12 ( 58.6%; 41.4 %,) |
NS |
| No of siblings | 7.6±4.5 | 7.3±4.5 | NS |
| Consanguinity (yes/no) |
14/15 (48.3%; 51.7%) |
11/18 (37.9%; 62.1%) |
NS |
BMI : Body Mass Index, SD: Standard Deviation
Table 1. Clinicoepidemiologic data of the studied children.
This statistically significant difference was also observed when comparison was made between patients and gender-matched controls (Table 2). Gender did not appear to affect melatonin level of SCA patients; there were no significant differences in the mean levels of melatonin in SCD males or females (Table 2).
| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Cases(n=14) | Controls (n=11) | P value | cases (n=15) | Controls (n=18) | P value | |
| Melatonin (pg/ml) | 17.5±20.7 | 154.5± 25.1 | 0.000 | 27.4±33.07 | 150.3±29.9 | <0.05 | |
| Cortisol (ng/ml) | 37.14±21.2 | 27.64±10.6 | 0.19 | 31.56±9.2 | 29.1±175 | 0.6 | |
| SCA patients | |||||||
| Variables | Males | Females | |||||
| Melatonin (pg/ml) | 17.46± 20.7 | 27.4±33.1 | 0.34 | ||||
| Cortisol (ng/ml) | 37.1± 21.2 | 29.1±17.5 | 0.27 | ||||
Data presented as means ± SD.
Table 2: Comparison of serum melatonin & cortisol in SCA patients and controls in relation to gender.
No significant differences in the mean levels of melatonin were observed between SCD patients on hydroxyurea (HU) therapy and those not receiving hydroxyurea (HU) (Table 3). No significant correlations were detected between melatonin and any of the patients clinical or laboratory finding However, correlations with age, vasoocclussive crisis, cortisol is negative but didn’t reach statistical significance (r=-0.02, p=0.9), (r=-0.13, p=0.5), (r= -0.28, p=0.15) respectively (Table 4).
| Variables | Patients receiving HU (n=11) | Patients not receiving HU (n=18) | P value |
|---|---|---|---|
| Melatonin (pg/ml) | 22.9±35 | 22.4±23.5 | 0.97 |
| Cortisol (ng/ml) | 40.6±20 | 28.3±18.1 | 0.1 |
HU: hydroxyurea, Data presented as means ± SD.
Table 3: Comparison of melatonin and cortisol in SCA patients receiving HU and SCA patients not receiving HU.
| Parameter | Melatonin | Cortisol |
|---|---|---|
| p-value | ||
| Age (years) | 0.9 | 0.9 |
| Age at diagnosis (years) | 0.49 | 0.41 |
| VOC: vaso-occlusive crisis frequency (times/year) | 0.5 | 0.38 |
| Weight (kg) | 0.45 | 0.93 |
| Height (cm) | 0.84 | 0.72 |
| BMI : body mass index (kg/m2) | 0.64 | 0.46 |
| Cortisol(ng/ml) | 0.15 | 0.15 |
Table 4: Correlation of serum melatonin and cortisol levels with patients clinical and laboratory value.
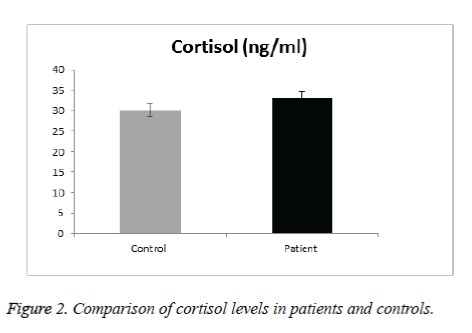
In Cortisol level, results didn’t show significant difference in SCA patients than in control group (Figure 2). Gender did not appear to affect cortisol level of SCA patients and there were no significant differences in the mean levels of cortisol in SCA males and females (Table 2). No significant difference was observed between cortisol and any of the patients clinical & laboratory data (p value>0.05) (Table 4).
Discussion
Sickle cell anemia is one of the diseases that exhibit circadian rhythm in their symptoms. A sufficient number of damaged erythrocytes cause the phenotype of SCD, characterized by hemolytic anemia and vaso-occlusion. In the present study we analyzed the effect of melatonin and cortisol on the sickle cell anemia. In particular, we explored the relation between serum levels of melatonin and cortisol in SCA and correlate with different disease parameters. The results indicated that there were no significant difference between patients and controls in regard to age, sex, anthropometric parameters, ethnicity, number of sibling and consanguinity. We found significantly reduced melatonin level in SCA patients compared to controls; this corroborates a previous study by Shimauti et al. that also found lower melatonin level in SCA patients than controls [23]. Furthermore, melatonin displays exceptional multiplicity of actions. Briefly, melatonin is involved in sleep initiation, vasomotor control, adrenal function, anti-excitatory actions, immunomodulation including anti-inflammatory properties, direct and indirect antioxidant actions, and energy metabolism [24,25]. Additionally, Chakravarty and Rizvi also showed in vivo a circadian modulation of human erythrocyte malondialdehyde and glutathione levels, as well as of membrane redox system and ascorbate free radical reductase activities, which emphasize the role of melatonin as an antioxidant and its function against oxidative stress in RBCs. Thus, many of melatonin actions are directly involved in the SCA pathophysiological process [26,27]. Similarly, the recent findings of Shimautiet al. showed that the SCA patients' mean age in their study was 21.3 years while the control group had a mean age of 29.2 years [23]. This is an indication that the disease contributes to the decrease in melatonin levels since a lower value would be expected in the older group as melatonin decreases along age [28]. Thus the above cited literatures are very well supported that the melatonin level is low because of the SCA itself. In our SCA patients' mean age was 10.4 ± 2.7 years while the control group had a mean age of 9.7 ± 2 years. Therefore a decrease in melatonin level in the control group was expected, in contrary when comparing the control group with the SCA patients there is a clear decrease of serum melatonin level in the SCA patients, confirming that the disease contributes to a significant decrease in melatonin level. Reduced melatonin levels were observed in other chronic diseases such as bronchial asthma [29], and inflammatory bowel disease [30]. This could be attributed to the chronic inflammatory condition in these diseases & reactive oxygen species production in these diseases.
The mean serum melatonin levels in the control group were 151.9 pg/mL. Plasma melatonin levels ranging from 400 to 500 pmol/mL was found by Kennaway and Voultsios [31], which corresponds to ~90 to 120 pg/mL [23]. Our results are higher because our controls from the pediatric age group. Previous review about melatonin done by Karasek [28] suggests that serum melatonin concentration reach about 180 pg/mL in children between 5 and 10 years old, and about 110 pg/mL between 15 and 35 years old. The mean age of the studied subjects is 10.4 year, age range from 6-16years, thus confirming that serum melatonin levels found in the present study are within the expected concentration for human blood Ueno-Towatari et al. [32] found a mean melatonin level in the Japanese population of ~90 pg/mL, with individuals reaching 220 pg/mL at 7:00 am., It should also be noted that there are no previous reports for serum melatonin levels in the Saudi population, also taking into consideration that our sample was collected from Al Madinah Al Munawarrah, which is genetically mixed. Melatonin is a hormone that supports a healthy immune system, proper sleep and is the main hormone involved in synchronizing the daily cycle of light exposure and physical activity in the body. While cortisol is critical for maintaining energy and supporting a healthy immune system. Hence both are the major hormones of the circadian system. For Cortisol level, results didn’t show significant difference in SCA patients than in control group. Gender did not appear to affect cortisol level of SCA patients and there were no significant differences in the mean levels of cortisol in SCD males and females. No gender difference in the secretion of melatonin and cortisol has been found in prepubertal children [25]. No significant difference was observed between cortisol & any of the patients clinical & laboratory data. There was no significantly lower basal morning cortisol in patients than controls (mean value in male patients were 37.14 ± 21.18 ng/ml compared with 27.64 ± 10.61ng/ml in control group with p value of 0.19), whereas in female patients 31.56 ± 9.2 ng/ml compared with 29.1 ± 175 ng/ml in control group with p value 0.6).
These present findings are also well supported by the earlier work of Saad and Saad who had observed that the 14 sickle cell anaemia patients and 16 normal controls were submitted to rapid ACTH stimulation test for basal cortisol determination. No significant differences were observed between the two groups for the basal and stimulated cortisol levels or for the increment at 30, 50 and 120 min. after infusion [33]. On other hand, Hagag et al. reported that 60 children with sickle cell anaemia with their age ranging from 5-17 years and mean age value of 13 ± 3 in comparison with 30 healthy children matched for age and sex as a control group. There was significantly lower basal morning cortisol in patients than controls (mean value in patients were 8.78 ± 3.5 ug/dl compared with 11.79 ± 2.3 μg/dl in control group [34]. As previously mentioned, previous animal studies emphasize that melatonin has the ability to decrease serum levels of corticosterone and also attenuates its level after acute and chronic stress [19,20]. In our study melatonin is negatively correlated with cortisol but didn’t reach statistical significance. In contrary in healthy young humans, single or repeated administration of melatonin did not affect basal or pharmacologically stimulated cortisol secretion [21]. Recent studies have investigated melatonin as antioxidant therapy for SCA treatment [35]. In our SCA patients, melatonin showed a potential role for detracting symptoms associated with SCA as it is correlated negatively with vaso-occlusive crisis (VOC) and cortisol but didn’t reach statistical significance. The exact mechanism of how melatonin and cortisol affect reactive oxygen species and its role in oxidative stress is not well understood. We cannot offer an explanation to why melatonin is more linked to SCA than cortisol (at least in our population). The lower serum melatonin levels in SCA patients may be due to the oxidation of melatonin by ROS and/or its consumption by free iron released by erythrocyte haemolysis, but other unknown process can also contribute to this result, including a decrease in melatonin secretion by the organism. Based on our findings, A larger longitudinal study is recommended with measuring both melatonin and cortisol levels at different times of the day before and after pharmacologically stimulated cortisol secretion. Up to our knowledge this study is the first one detecting serum level of melatonin in children in Saudi Arabia. We recommend the addition of melatonin for the treatment regimen of SCA.
Acknowledgments
The author (s) thank Deanship of Scientific Research (DSR), Taibah University, Madinah, Saudi Arabia for funding the project grant no. 6102/1435.Madinah Maternity and Children Hospital (MMCH) & all the children and their parents who so willingly participated in this study. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Deanship of Scientific Research (DSR), Taibah University, Madinah, Saudi Arabia, grant no. 6102/1435. The authors are grateful for DSR financial support.
References
- Taylor JG, Ackah D, Cobb C, Orr N, Percy MJ, Sachdev V, Machado R, Castro O, Kato GJ, Chanock SJ, Gladwin MT. Mutations and polymorphisms in hemoglobin genes and the risk of pulmonary hypertension and death in sickle cell disease. Am J Hematol 2008; 83: 6-14.
- Embury SH, Mohandas N, Paszty C, Cooper P, Cheung AT. In vivo blood flow abnormalities in the transgenic knockout sickle cell mouse. J Clin Invest 1999; 103: 915-920.
- Ataga KI, Key NS. Hypercoagulability in sickle cell disease: new approaches to an old problem. Hematology Am SocHematolEduc Program 2007; 91-6.
- Wun T, Paglieroni T, Tablin F, Welborn J, Nelson K, Cheung A. Platelet activation and platelet-erythrocyte aggregates in patients with sickle cell anemia. J Lab Clin Med 1997; 129: 507-516.
- Powars DR, Elliott-Mills DD, Chan L, Niland J, Hiti AL, Opas LM, Johnson C. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med. 1991; 115: 614-620.
- El-Ghamrawy MK, Hanna WM, Abdel-Salam A, El-Sonbaty MM, Youness ER, Adel A. Oxidant-antioxidant statusin Egyptian children with sickle cell anemia: a single center based study. J Pediatr (Rio J) 2014; 90: 286-292
- Tan DX, Manchester LC, Terron PP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res 2007; 42: 28-42.
- Sener G, Tugtepe H, Velioglu-Ogunc A, Cetinel S, Gedik N, YegenBC. Melatonin prevents neutrophil-mediated oxidative injury in Escherichia coli-induced pyelonephritis in rats. J Pineal Res. 2006; 41: 220-227.
- Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. ActaBiochim Pol. 2003; 50: p. 1129-46.
- Wang L, Muxin G, Nishida H, Shirakawa C, Sato S, Konishi T. Psychological stress-induced oxi-dative stress as a model of sub-healthy condition and the effect of TCM. Evidence Based Complementary and Alternative Medicine 2007; 4: 195-202.
- Cuzzocrea S, Reiter RJ. Pharmacological actions of melatonin in acute and chronic inflammation. Curr Top Med Chem 2002; 2: 153-165.
- Gizi A, Papassotiriou I, Apostolakou F, Lazaropoulou C, Papastamataki M, Kanavaki I, Kalotychou V, Goussetis E, Kattamis A, Rombos I, Kanavakis E. Assessment of oxidative stress in patients with sickle cell disease: The glutathione system and the oxidant-antioxidant status. Blood Cells Mol Dis 2011; 46: 220-225.
- Silva DG, Belini Junior E, de Almeida EA, Bonini-Domingos CR. Oxidative stress in sickle cell disease: an overview of erythrocyte redox metabolism and current antioxidant therapeutic strategies. Free RadicBiol Med 2013; 65: 1101-1109.
- Wassmer E, Whitehouse WP. Melatonin and sleep in children with neurodevelopmental disabilities and sleep disorders. Current Paediatrics 2006; 16: 132–138.
- Gamble KL, Berry R, Frank SJ, Young ME .Circadian clock control of endocrine factors. Nat Rev Endocrinol 2014; 10: 466-475.
- James FO, Walker CD, Boivin DB. Controlled exposure to light and darkness realigns the salivary cortisol rhythm in night shift workers. ChronobiolInt 2004; 21: 961-972.
- Horowitz TS, Cade BE, Wolfe JM, Czeisler CA. Efficacy of bright light and sleep/darkness scheduling in alleviating circadian maladaptation to night work. Am J PhysiolEndocrinolMetab 2001; 281: E384-E391.
- Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms 2003; 18: 513-523.
- Konakchieva R, Mitev Y, Almeida OF, Patchev VK. Chronic melatonin treatment and the hypothalamo-pituitary-adrenal axisin the rat: attenuation of the secretory response to stress an effects on hypothalamic neuropeptide content and release. Biol Cell 1977; 89: 587–596.
- Torres-Farfan C, Richter HG, Rojas-Garcia P, Vergara M, Forcelledo ML, Valladares LE, Torrealba F, Valenzuela GJ, Seron-Ferre M. Melatonin receptor in the primate adrenal gland: inhibition of adrenocorticotropin-stimulated cortisol production by melatonin. J ClinEndocrinolMetab 2003; 88: 450-458
- Perras B, Ozcan S, Fehm HL, Born J . Melatonin does not inhibit hypothalamic-pituitary-adrenal activity in waking young men. J Neuroendocrinol 2005; 17: 811-816
- Darbari DS, Onyekwere O, Nouraie M, Minniti CP, Luchtman-Jones L, Rana S. Markers of severe vaso-occlusive painful episode frequency in children and adolescents with sickle cell anemia. J Pediatr. 2012; 160: 286-290.
- Shimauti ELT, DaniloGrunigHumberto Silva, Eduardo Alves de Almeida, Paula Juliana AntoniazzoZamaro, EdisBelini Junior, Claudia Regina Bonini-Domingos. Serum melatonin level and oxidative stress in sickle cell anemia. Blood Cells, Molecules, and Diseases 2010; 45: 297-301.
- Hardeland R. Melatonin and the theories of aging: a critical appraisal of melatonin's role in antiaging mechanisms. J Pineal Res 2013; 55: 325-56.
- Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev 2005; 9: 11-24.
- Chakravarty S, Rizvi SI. Circadian modulation of human erythrocyte plasma membrane redox system by melatonin. NeurosciLett 2012; 518: 32-35.
- Chakravarty S, Rizvi SI. Day and Night GSH and MDA Levels in Healthy Adults and Effects of Different Doses of Melatonin on These Parameters. Int J Cell Biol 2011; 1-5.
- Karasak M. Melatonin, human aging, and age-related diseases. ExpGerontol 2004; 39: 1723-1729.
- Gumral N, NazirogluM, Ongel K, Beydilli ED, Ozguner F, Sutcu R. Caliskan S, Akkaya A. Melatonin levels and enzymatic antioxidant defense system decrease in blood of patients with bronchial asthma. ToxicolInd Health 2009; 25: 411-416.
- Mozaffari S, Abdollahi M. Melatonin, a Promising Supplement in Inflammatory Bowel Disease Current Pharmaceutical Design 2011; 17: 4372-4378.
- Kennaway DJ, Voultsios A. Circadian rhythm of free melatonin in human plasma. J Clin. EndocrinolMetab 1998; 83: 1013-1015.
- Ueno-Towatari T, Norimatsu K, Blazejczyk K, Tokura H, Morita T. Seasonal variations of melatonin secretion in young females under natural and artificial light conditions. J PhysiolAnthropol 2007; 26: 209-215.
- Saad ST, Saad MJ. Normal cortisol secretion in sickle cell anemia. Trop Geogr Med 1992; 44: 86-88.
- Hagag AA, Elmashad G, Abd El-Lateef AE. Clinical significance of assessment of thrombospondin and placenta growth factor levels in patients with sickle cell anemia: two centersegyptian studies. Mediterr J Hematol Infect Dis 2015; 9: 60-66.
- Da Silva DG, Ricci OA, De Almeida EA, Bonini-Domingos CR. Potential utility of melatonin as an antioxidant therapy in the management of sickle cell anemia. Journal of pineal research. 2015; 58: 178-188.