ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Special Issue Article - Biomedical Research (2017) Health Science and Bio Convergence Technology: Edition-II
Anti-physical fatigue effect of polysaccharides from Lepidium meyenii. Walp. and the possible mechanisms
Rong-Wei Li1, Jun-Cong He2* and Dong-Han Song3
1Department of Physical Education, Hebei Normal University of Science and Technology, Qinhuangdao, PR China
2Department of Physical Education, Yunnan University of Finance and Economics, Kunming, PR China
3Dalian Campus, Shenyang Conservatory of Music, Dalian, PR China
- *Corresponding Author:
- Jun-Cong He
Department of Physical Education
Yunnan University of Finance and Economics, PR China
Accepted date: March 19, 2017
Lepidium meyenii. Walp. (maca), a Peruvian plant, has been utilized in the Andean region as a food and a medicine for centuries. This study was carried out to investigate the anti-physical fatigue effect of polysaccharides from maca (MCP) and the possible mechanisms. Mice were administered daily doses of 500, 1000 and 2000 mg/kg MCP for 28 days. After the treatment period, the anti-fatigue effect of MCP were evaluated by exhaustive swimming exercise and several biochemical parameters related to physical fatigue were determined: blood lactic acid (BLA), serum urea nitrogen (SUN), glycogen, superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), and malondialdehyde (MDA). Results showed that MCP could prolong exhaustive swimming times, decrease the levels of BLA and SUN, and increase the liver glycogen contents. Furthermore, MCP increased activities of SOD, GPx and CAT, and decreased MDA level. The present findings indicate that MCP has obvious anti-physical fatigue effect, and the possible mechanisms were to delay the increase of BLA, reduce the catabolic decomposition of protein for energy and lipid peroxidation, and improve reserve and antioxidant enzymes activities.
Keywords
Anti-physical fatigue, Lepidium meyenii, Polysaccharides, Exhaustive swimming exercise, Mechanisms
Abbreviations
MCP: Polysaccharides from Maca; BLA: Blood Lactic Acid; SUN: Serum Urea Nitrogen; SOD: Superoxide Dismutase; GPx: Glutathione Peroxidase; CAT: Catalase; MDA: Malondialdehyde; ROS: Reactive Oxygen Species
Introduction
Fatigue is a complex phenomenon that can be defined as the inability to maintain expected muscle strength, leading to reduced performance during prolonged exercise [1]. There are several theories regarding the mechanism of physical fatigue, such as radical theory, mutation theory, exhaustion theory, clogging theory, homeostasis theory and protective inhibition theory, etc. [2]. Among the physical fatigue mechanisms, the radical theory has been attracting more interest. Strenuous exercise can lead to the production and accumulation of excess reactive free radicals, and free radicals cause oxidative damage to nucleic acids, proteins, carbohydrates and lipids. which results in oxidation stress injury to the body [3]. Several studies have shown that antioxidant supplementation could elevate the endurance capacity and facilitate recovery from fatigue [4,5].
Lepidium meyenii Walp. commonly known as maca, is a Peruvian plant that belongs to the Brassicaceae family (Cruciferae). It is mainly domesticated at altitudes between 3500 and 4500 m above sea level in the central Andean region of Peruvian [5]. Maca has been utilized in the Andean region as a food and a medicine for centuries. As a food source, maca displays a high nutritional value and is rich in sugars, proteins, starches, and minerals [6]. Medicinally, it has been used to enhance both female and male fertility, improve sexual dysfunction, prevent osteoporosis and relieve menopausal syndrome [7]. It is also recommended in traditional medicine as being effective in cancer prevention and treatment, anemia, gastritis, high blood pressure, and depression [8]. Pharmacological studies demonstrated that maca extracts possess diverse biological activity, such as antioxidant, antidepressant, antiviral, anti-fatigue, antihyperlipidemic, hypoglycemic, improving sexual performance, enhancing fertility, spermatogensis, and prevention of estrogen-deficient bone loss [6,9]. Previous studies on biologically active substance from maca mainly focused on phytosterols, alkaloids, isothiocyanates, glucosinolates, macaenes, and macamides [10]. However, recent studies have also shown that polysaccharides from maca (MCP) exhibit strong antioxidant activities [11], which suggest that MCP might be possible to reduce free radical cause oxidative damage and physical fatigue. The present study was undertaken to investigate the anti-physical fatigue effect and possible mechanism of MCP by exhaustive swimming exercise of mice.
Materials and Methods
Plant material
Yellow maca was harvested from the Lijiang area (Connection area of Yunnan-Guizhou Plateau and the Qinghai-Tibet Plateau of China, >3900 m above sea level) in 2014. The roots of maca were collected, dried (the average weight of dried roots of maca is about 6.5 g), and purchased from the Yunnan Chinese Herbal Medicine Company (Kunming, China).The identity of the plant was authenticated by Professor Wang LM, Institute of Medicinal Plant of CAS (Kunming, China). Voucher specimens were deposited at the herbarium of medicinal plant of CAS.
Chemicals and reagents
The commercial diagnostic kits for the determination of blood lactic acid (BLA), serum urea nitrogen (SUN), liver glycogen, superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), and malondialdehyde (MDA) were purchased from Jiancheng Biologic Project Company (Nanjing, China). All other chemicals and reagents were of analytical grade and were obtained from the usual commercial sources.
Preparation of polysaccharides from maca
Polysaccharides from maca (MCP) were prepared as described previously [11] with slight modifications. In brief, dried roots of maca were ground in a blender for 10 s to produce powder with an approximate size of 1 mm. Then the powder was extracted with distilled water in an ultrasonic cleaner bath set at a selected extraction conditions (solid/liquid ratio of 1:25, extraction time of 20 min, ultrasonic power of 200 W and extraction temperature of 50°C). After filtration to remove debris fragments, the filtrate was concentrated by rotary vacuum evaporator at 60°C, and subsequently was dried with a spray dryer. A certain amount of aqueous extract of maca was dissolved in 200 mL water, and 2 mL 10% (w/v) amylase and 1 mL 0.2 Mphosphate buffer (pH 6.5) was added then. After enzymatic hydrolysis in water bath at 50°C for 2 h, 1 mL glucoamylase was added and incubated for 1 h. Enzymatic solution was heated to 100°C rapidly to inactivate enzyme reaction, and then cooled and diluted to 250 mL. The diluents extract solution was centrifuged (2,000 × g, 30 min), then the supernatant was precipitated with addition of 4 times volume 95% (v/v) ethanol, and the precipitate was collected after centrifugation, redissolved in distilled water and treated with Sevag’s reagent several times to remove protein and then dialyzed against distilled water for 48 h at 4°C. MCP was again precipitated with ethanol and the precipitate thus obtained was lyophilized. The polysaccharides content was determined by the phenol-sulphuric acid method using glucose as a standard. Extraction yield of MCP was calculated as the polysaccharides content of extraction divided by dried roots of maca weight, and the calculated extraction yield of MCP was 2.37% (w/w).
Chemical properties of MCP
The chemical properties of MCP were preliminary identified by different chemical reactions. Purple ring phenomenon was observed in the molish reaction, and orange-red color phenomenon was observed in the phenol-sulfuric acid reaction, which indicates that MCP belongs to the polysaccharide. Precipitation phenomenon wasn't observed in the Fehling reagent reaction, which indicates that MCP doesn't contain reducing sugar. Purple phenomenon was observed in the carbazole and sulphuric acid reaction, which indicates that MCP contains uronic acid. Blue-violet phenomenon was observed in the ninhydrin reaction, which indicates that MCP contains protein. Color change wasn't observed in the iodinepotassium iodide reaction, which indicates that MCP doesn't contain starch substances. The above data show that MCP is an acidic polysaccharide, which contains protein, and doesn't contain starch and reducing sugar.
Animals and grouping
Male Kunming mice (18-22 g) were purchased from the Experimental Animal Center of Kunming Medical University (Kunming, China) with certificate number: SYXK (Ji) 2015-0127. The animals were housed at a temperature of 21-23°C and a relative humidity of 45-55 % and were submitted to a 12 h light/dark cycle. The mice had unrestricted access to tap water and standard rodent diet. All animal handling procedures were performed in strict accordance with the China legislation of the use and care of laboratory animals, and were approved by the Institutional Animal Ethics Committee (IAEC) of Yunnan University of Finance and Economics (Kunming, China) (license number: UNUV2015027). Every effort was made to minimize the number of used animals and their suffering.
Following an adaptation period of a week, 48 animals were randomly divided into four groups of twelve mice each: control (C), low-dose MCP-treated (MCP-L), middle-dose MCPtreated (MCP-M) and high-dose MCP-treated (MCP-H) groups. The mice of control group received an oral administration of distilled water in a volume of 1.0 mL, and the mice of treated groups received the same volume of MCP (500, 1000 and 2000 mg/kg bodyweight) once a day for 28 consecutive days.
Exhaustive swimming exercise
Exhaustive swimming exercise was applied in this study to evaluate the anti-fatigue effect of MCP. Thirty minutes after the last treatment with MCP or distilled water, the mice performed the forced swimming exercise as described previously [12]. Briefly, the mice were dropped individually into acrylic plastic tank (50 cm × 50 cm × 40 cm) containing water at 25 ± 0.5°C (30 cm deep). A tin wire (4% of body weight) was loaded on the tail root of the mouse. Mice were regarded as being exhausted when they stayed in the water for 10 s [13], and their exhaustive swimming time was immediately recorded.
Analysis of biochemical parameters related to physical fatigue
After the completion of the swimming exercise, the mice were anaesthetized using ethyl ether and sacrificed by exsanguination via the abdominal aorta. Blood samples were collected and serum was separated by centrifugation (2,000 × g, 10 min) at 4°C for the BLA and SUN analyses. Next, the liver were carefully removed and rinsed in ice cold physiological saline solution (0.9% (v/v) NaCl), blotted dry and stored at -80°C until required for the glycogen, SOD, GPx, CAT and MDA analyses. All the biochemical parameters were measured with an automated biochemistry analyzer using commercial diagnostic kit according to the manufacturer’s recommended procedures.
Statistical analysis
The statistical analysis was performed using the statistical package for the Social Sciences (version18.0; SPSS, Chicago, IL, USA) All tests were conducted in triplicate. Results were expressed as mean ± standard deviation (SD) and were analyzed using the analysis of variance (ANOVA) and Student’s t-test. p values<0.05 were regarded as having statistical significance.
Results and Discussion
Effects of MCP on the body weights of mice
Effects of MCP on the body weights of mice are shown in Figure 1. Before on the experiments (0 days), all the groups had no significant difference in body weights (P>0.05). After 7 days, 14 days, 21 days, and 28 days of treatment with MCP, all the groups also had no significant difference in body weights (p>0.05). So MCP had no significant effect on the body weights of mice.
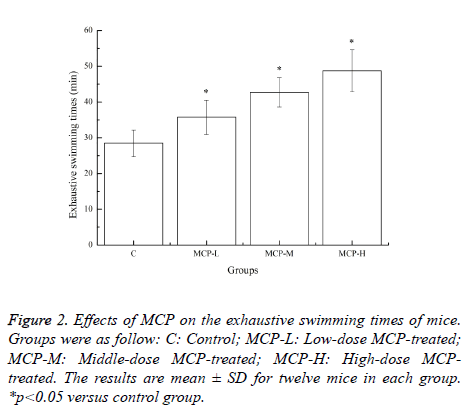
Effects of MCP on the exhaustive swimming times of mice
Swimming was chosen as a suitable model since it is a natural behaviour of rodents. The method causes less mechanical stress and injury, and may minimize the magnitude of injury caused due to the generation of free radicals and reactive oxygen species (ROS) [14]. The improvement of exercise endurance is the most powerful macro representation of antifatigue enhancement [15]. Swimming to exhaustion is a direct measure reflecting objectively the exercise endurance of body. Effects of MCP on the exhaustive swimming times of mice are shown in Figure 2. After 28 days of treatment with MCP, exhaustive swimming times in the MCP-L, MCP-M and MCPH groups were significantly prolonged compared with that in the C group (p<0.05). The results clearly indicated that MCP has obvious anti-physical fatigue effect and the effect was significantly dose-dependent.
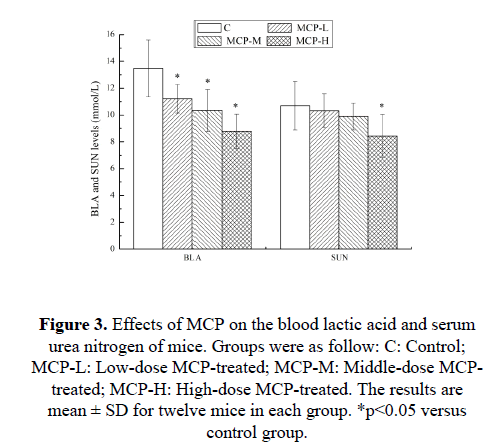
Effects of MCP on the blood lactic acid and serum urea nitrogen of mice
It is known that the BLA and SUN are important blood biochemical parameters related to fatigue. BLA is a product of glycolysis under anaerobic condition. Glycolysis is the primary form of metabolism used during strenuous exercise in a short time. The increased BLA production results in decreasing the internal pH value, which may lead to impairment of muscle contraction and harm organs [16]. The BLA accumulation has been considered as a major inducer of fatigue, and the inhibition of BLA accumulation or reduction of BLA level represents an anti-fatigue effect [17]. SUN is a sensitive index to evaluate the bearing capability when human bodies suffer from a physical load. There is a positive correlation between the SUN in vivo and exercise tolerance. In other words, the worse the body is adapted for exercise tolerance, the more significantly the urea nitrogen level increases [18]. Effects of MCP on the BLA and SUN of mice are shown in Figure 3. After 28 days of treatment with MCP, compared with the C group, the BLA levels in the MCP-L, MCP-M and MCP-H groups, as well as the SUN levels in the MCP-H groups, were significantly lower (p<0.05). The results indicated that MCP can effectively delay the increase of BLA, reduce the catabolic decomposition of protein for energy, and ameliorate physical fatigue.
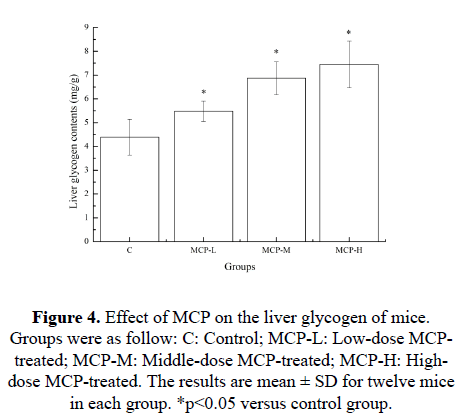
Effects of MCP on the liver glycogen of mice
Muscle glycogen content is exhausted after strenuous exercise, and energy is then derived from circulating glucose released from the liver via breakdown of glycogen [17]. The increasing of glycogen stored in liver is advantage to enhance the exercise endurance. So, liver glycogen is an important factor in the development of fatigue [18]. Effects of MCP on the liver glycogen of mice are shown in Figure 4. After 28 days of treatment with MCP, compared with the C group, the liver glycogen contents in the MCP-L, MCP-M and MCP-H groups were significantly higher (p<0.05). The results indicated that increased level of liver glycogen may be one of the pathways behind the MCP’s anti-physical fatigue effect.
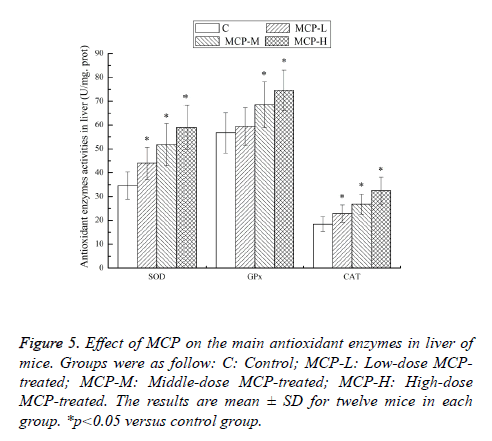
Effects of MCP on the main antioxidant enzymes in liver of mice
Strenuous exercise is often associated with an increase in the production of free radicals and ROS in various tissues, which may overwhelm the capacity of the antioxidant defense systems, and the result can lead to increased oxidative stress [16]. It is well documented that oxidative stress plays a very important role in the muscle fatigue, muscle damage and physical performance. Main antioxidant enzymes of the living body include SOD, GPx and CAT. SOD catalyzes the dismutation of superoxide into oxygen and hydrogen peroxide. GPx is a selenoenzyme which catalyzes the reduction of hydroperoxides at the expense of reduced glutathione. CAT catalyzes the breakdown of hydrogen peroxide to form water [13]. These antioxidant enzymes become weaker during fatigue conditions. So, the improvement antioxidant enzymes activities can help to fight against physical fatigue [19]. The liver is an important organ of the organism. Changes of antioxidant enzyme in liver are more obvious than that in other tissues [12,20]. Effects of MCP on the main antioxidant enzymes activities of mice are shown in Figure 5. After 28 days of treatment with MCP, compared with the C group, the SOD and CAT activities in the MCP-L, MCP-M and MCP-H groups, as well as the GPx activities in the MCP-M and MCP-H groups, were significantly higher (p<0.05). The results clearly indicated that MCP was able to up-regulate antioxidant enzymes activities to protect against oxidative stress-induced injury during strenuous exercise and defend physical fatigue.
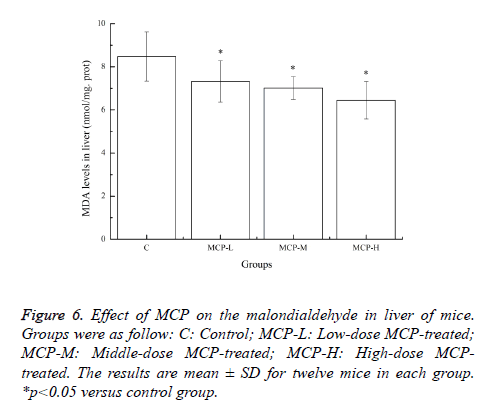
Effects of MCP on the malondialdehyde in liver of mice
When free radicals are generated they can attack polyunsaturated fatty acids in the cell membrane leading to a chain of chemical reactions called lipid peroxidation [21]. It has been reported that strenuous exercise produces an increase in the markers of lipid peroxidation in target tissues and blood [22]. MDA, the end product of lipid peroxidation, is a good marker of free radical-mediated damage and oxidative stress [13]. Effects of MCP on the MDA levels of mice are shown in Figure 6. After 28 days of treatment with MCP, compared with the C group, the MDA levels in the MCP-L, MCP-M and MCP-H groups were significantly lower (p<0.05). The results clearly indicated that MCP could reduce lipid peroxidation and ameliorated physical fatigue.
Conclusion
The results of the study found that MCP could prolong exhaustive swimming time and had obvious anti-physical fatigue effect. These results also suggested that the possible mechanisms of MCP’s anti-physical fatigue effect were to delay the increase of BLA, reduce the catabolic decomposition of protein for energy and lipid peroxidation, and improve liver glycogen reserve and antioxidant enzymes activities. This study provides evidence that supports the use of MCP as an effective ergogenic aid for competing athletes. Future research should go to explore the relationship between MCP with different molecular weights fractions and anti-physical fatigue effect, and analyze monosaccharide composition and glycosidic linkage of MCP.
Acknowledgement
This study was supported by grants from the Research Project of Yunnan University of Finance and Economics (No. YNU2014132x).
References
- Kim DI, Kim KS. Walnut extract exhibits anti-fatigue action via improvement of exercise tolerance in mice. Lab Anim Res 2013; 29: 190-195.
- Wang X, Xing R, Chen Z, Yu H, Li R, Li P. Effect and mechanism of mackerel (Pneumatophorus japonicus) peptides for anti-fatigue. Food Funct 2014; 5: 2113-2119.
- Na C, Yoon SY, Kim JB, Na DS, Dong MS, Lee MY, Hong CY. Anti-fatigue activity of Hovenia dulcis on a swimming mouse model through the inhibition of stress hormone expression and antioxidation. Am J Chinese Med 2013; 41: 945-955.
- Tharakan B, Dhanasekaran M, Manyam BV. Antioxidant and DNA protecting properties of anti-fatigue herb Trichopus zeylanicus. Phytother Res 2005; 19: 669-673.
- Yábar E, Pedreschi R, Chirinos R, Campos D. Glucosinolate content and myrosinase activity evolution in three maca (Lepidium meyenii Walp.) ecotypes during preharvest, harvest and postharvest drying. Food Chem 2011; 127: 1576-1583.
- Bai N, He K, Roller M, Lai CS, Bai L, Pan MH. Flavonolignans and other constituents from Lepidium meyenii with activities in anti-inflammation and human cancer cell lines. J Agric Food Chem 2015; 63: 2458-2463.
- Liu H, Jin WW, Fu CH, Dai PF, Yu YT, Huo Q, Yu LJ. Discovering anti-osteoporosis constituents of maca (Lepidium meyenii) by combined virtual screening and activity verification. Food Res Int 2015; 77: 215-220.
- Pino-Figueroa A, Nguyen D, Maher TJ. Neuroprotective effects of Lepidium meyenii (Maca). Ann N Y Acad Sci 2010; 1199: 77-85.
- Sandoval M, Okuhama NN, Angeles FM, Melchor VV, Condezo LA, Lao J, Miller MJS. Antioxidant activity of the cruciferous vegetable Maca (Lepidium meyenii). Food Chem 2002; 79: 207-213.
- Valentová K, Buckiová D, Kren V, Peknicová J, Ulrichová J, Simánek V. The in vitro biological activity of Lepidium meyenii extracts. Cell Biol Toxicol 2006; 22: 91-99.
- Zha S, Zhao Q, Chen J, Wang L, Zhang G, Zhang H, Zhao B. Extraction, purification and antioxidant activities of the polysaccharides from maca (Lepidium meyenii). Carbohydr Polym 2014; 111: 584-587.
- Chen Z, Li SS, Wang XQ, Zhang CL. Protective effects of Radix Pseudostellariae polysaccharides against exercise-induced oxidative stress in male rats. Exp Ther Med 2013; 5: 1089-1092.
- Yan F, Wang B, Zhang Y. Polysaccharides from Cordyceps sinensis mycelium ameliorate exhaustive swimming exercise-induced oxidative stress. Pharm Biol 2014; 52: 157-161.
- Lin Y, Liu HL, Fang J, Yu CH, Xiong YK, Yuan K. Anti-fatigue and vasoprotective effects of quercetin-3-O-gentiobiose on oxidative stress and vascular endothelial dysfunction induced by endurance swimming in rats. Food Chem Toxicol 2014; 68: 290-296.
- Jin HM, Wei P. Anti-fatigue properties of tartary buckwheat extracts in mice. Int J Mol Sci 2011; 12: 4770-4780.
- Fu X, Ji R, Dam J. Antifatigue effect of coenzyme Q10 in mice. J Med Food 2010; 13: 211-215.
- Wu JY, Leung HP, Wang WQ, Xu C. Mycelial fermentation characteristics and anti-fatigue activities of a Chinese caterpillar fungus, Ophiocordyceps sinensis strain Cs-HK1 (Ascomycetes). Int J Med Mushrooms 2014; 16: 105-114.
- Tang W, Zhang Y, Gao J, Ding X, Gao S. The anti-fatigue effect of 20(R)-ginsenoside Rg3 in mice by intranasally administration. Biol Pharm Bull 2008; 31: 2024-2027.
- You LJ, Zhao MM, Regenstein JM, Ren JY. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Food Chem 2011; 124: 188-194.
- Chen QP, Wei P. Icariin supplementation protects mice from exercise-induced oxidant stress in liver. Food Sci Biotechnol 2013; 22: 1 5.
- Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003; 189: 41-54.
- Morillas-Ruiz JM, Villegas García JA, López FJ, Vidal-Guevara ML, Zafrilla P. Effects of polyphenolic antioxidants on exercise-induced oxidative stress. Clin Nutr 2006; 25: 444-453.